Kinetics and Thermochemistry review
1/14
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
15 Terms
Increase in temperature
Increase reaction rate, increase collision frequency, increase kinetic energy of collisions, no change in activation energy
Increase in concentration
Decreased reaction rate, decrease collision frequency, no change kinetic energy of collisions, no change in activation energy
Addition of catalyst
Increase reaction rate, no change in collision frequency, no change in kinetic energy of collisions, decrease in activation energy
Increased surface area
Increase reaction rate, increase collision frequency, no change in kinetic energy of collisions, no change in activation energy
Low specific heat
Heats up and cools down quicker
High specific heat
Heats up and cools down slower
1 Calorie
1000 calories
Endothermic energy
Positive 🔼H, feels cold, absorbs energy, energy flows from surroundings to system
Exothermic reactions
Negative 🔼H, feels hot, releases energy, energy flows from system to surroundings
Temperature
Measure of average kinetic energy
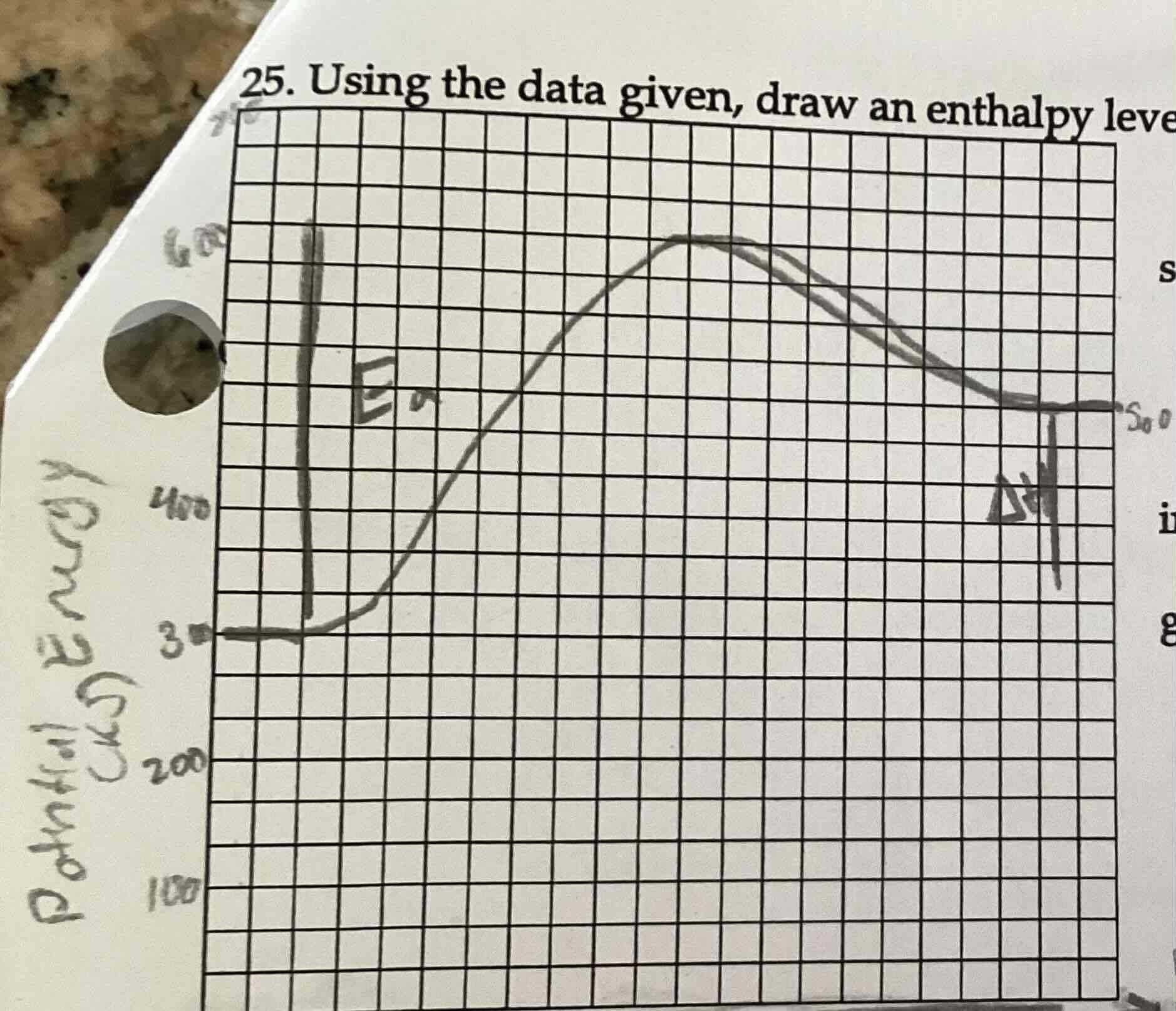
Endothermic (feel cold) or exothermic (feel hot)
Endothermic
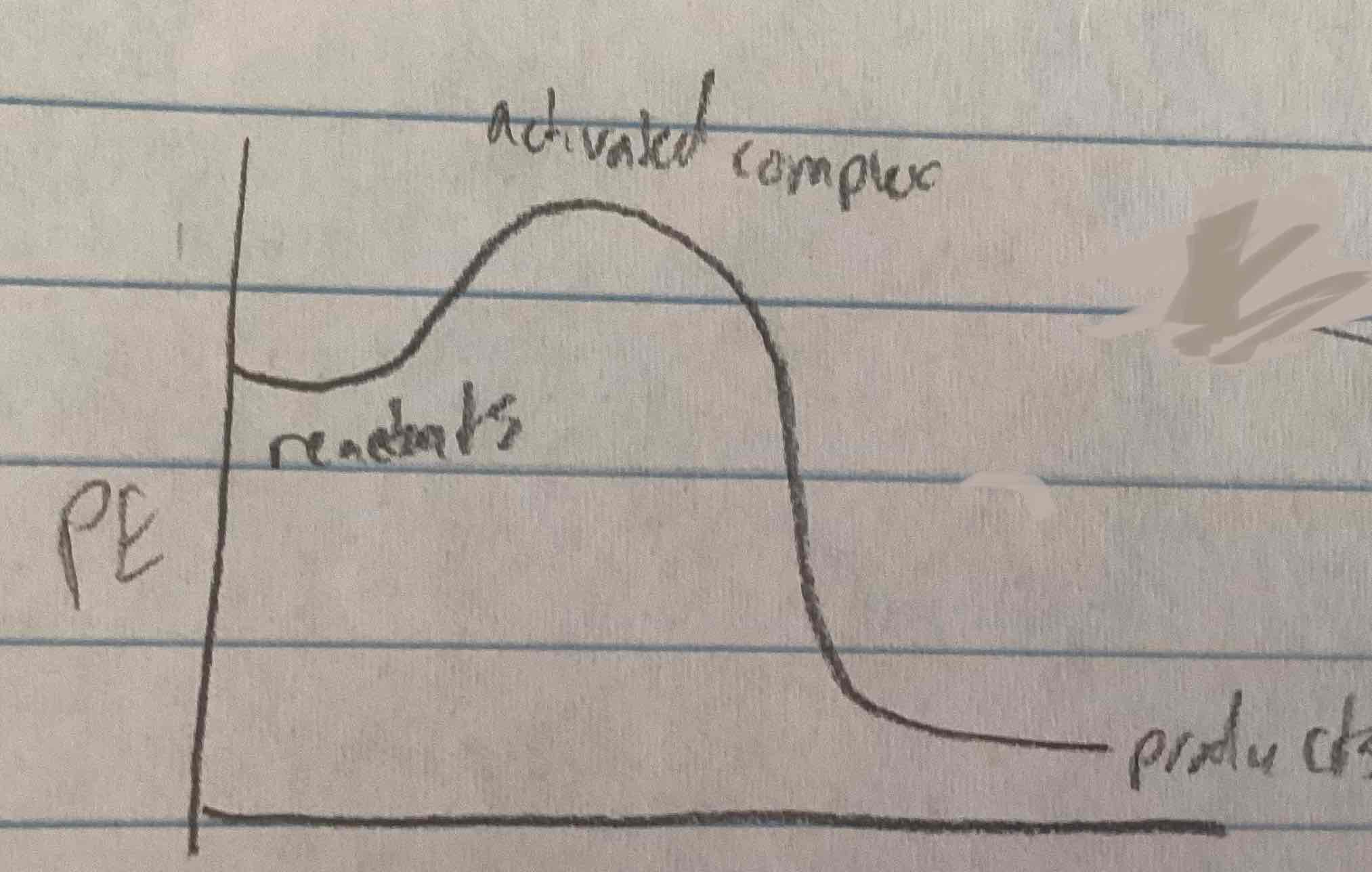
Endothermic (feels cold) or exothermic (feels hot)
Exothermic
activation energy on a diagram
Activated complex - PE of reactants
🔼H on a diagram
PE of products - PE of reactants
Specific heat of water
4.18 J/gC