MICR:3164 Antimicrobial Therapies and Drug Resistance
1/48
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
49 Terms
antimicrobial chemotherapy
deliver a drug to an infected person and kill the microbial cells without harming the host cells
types of drug activity for antimicrobials
broad-spectrum
narrow-spectrum
antibiotics/antibacterial
antimicrobial
prophylaxis
broad-spectrum
effective against a wide variety of microbial types
narrow-spectrum
effective against a limited array of microbial types
antibiotics/antibacterial
substances that can inhibit or destroy microbes; generally bacteria
antimicrobial
inclusive term for any antimicrobial drug
prophylaxis
used to prevent infection of a person at risk
before starting antimicrobial treatment, one needs to?
identify the microbe(s) causing disease
determine the susceptibility of the microbe to antimicrobials
examine the condition and history of the patient
tests for susceptibility
kirby-bauer
e-test
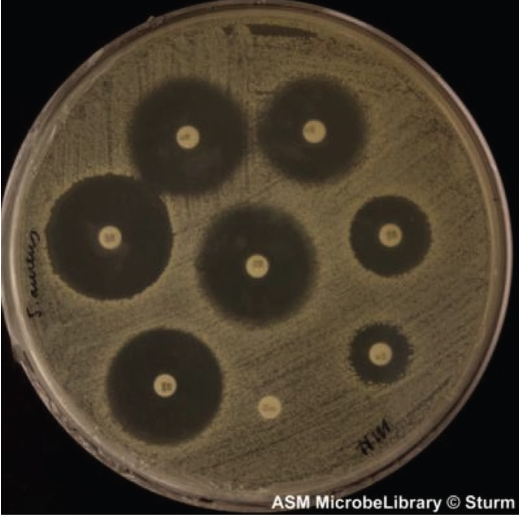
kirby-bauer
bacteria of interest are spread on plate
discs with antimicrobial drugs are placed on top
incubate, then measure zone of inhibition around the discs and compared to a standard
E-test
bacteria of interest are spread on plate
strip of plastic with a gradient of antimicrobial concentrations is placed on top
after 48 hours of incubation, look for susceptibility
MIC

minimum inhibitory concentration (MIC)
the smallest concentration of drug that visibly inhibits growth
therapeutic index
compares the toxic dose to the minimum effective dose
toxic dose / MIC
bigger TI, better and safer
why might treatment not work?
drug cannot reach infected area (eg joints, brain)
resistant microbes were missed during testing
more than one pathogen is responsible for the disease
patient didn’t take antimicrobials as prescribed
impact of patient history
pre-existing conditions
allergies to antimicrobials
disease of kidneys or liver
age, pregnancy
reactions with other medications
how can we target microbes without damaging host cells?
attack structure and pathways specific to microbial cells
disrupt the structure of function to the point where the microbe can no longer survive
drug action mechanisms
inhibit cell wall synthesis
inhibit nucleic acid structure and function
inhibit protein synthesis
interfere with cytoplasmic membrane
inhibit folic acid synthesis
drugs that target cell wall
beta-lactam antibiotics
vancomycin
beta-lactam antibiotics
ex. penicillins, cephalosporins, carbapenems
block the last stage of cross-linking peptides in peptidoglycan synthesis
more effective on gram-positive bacteria
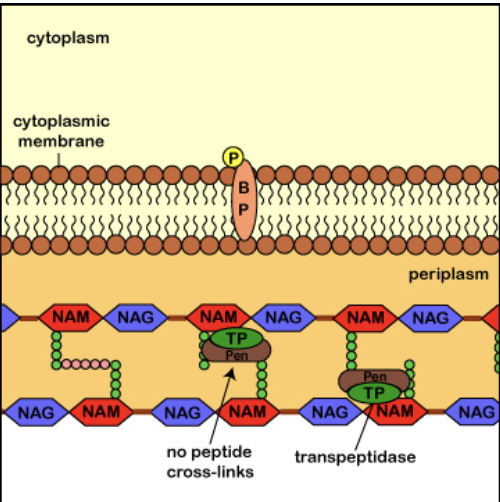
vancomycin
prevents proper peptidoglycan production
important for role in treating methicillin-resistant Staphylococcus aureus (MRSA)
drugs that target nucleic acids
quinolone antibioticsq
quinolone antibiotics
ex. ciprofloxacin
inhibit DNA transcription, replication, necessary enzymes
drugs that target protein synthesis
ex. tetracyclines, clindamycin
block successful translation at the ribosome/mRNA level
drugs that target cytoplasm membrane
ex. polymyxin, daptomycin
interact with phospholipids, causing distortion and leakage of cell contents
more effective on gram-negative bacteria
drugs that target folic acid synthesis
sulfonamides
sulfonamides
ex. sulfamethoxazole, silver sulfadiazene
inhibit enzymes required for folic acid synthesis
what is folic acid?
an essential vitamin required for DNA synthesis and cell replication
bacteria synthesize folic acid, humans require it in diet
why is treatment for fungal infections more complicated?
most antimicrobials were designed for prokaryotic cells
similarities between fungal and human cells makes treatments more toxic
how do we treat fungal infections?
target fungal membranes, sterols, cell wall, and enzymes
ex. amphotericin B, fluconazole
treating protozoal infections
diversity of these microbes = diversity of treatment
quinine
metronidazole (Flagyl)
quinine
antimalarial compound, now replaced with chloroquine and primaquine (less toxic)
metronidazole (Flagyl)
an amoebicide with many uses
giardiasis, trichomoniasis, some anaerobic bacterial infections
treating helminth infections
most difficult to treat because most similar to humans
blocking reproduction doesn’t eliminate adult worms
goal to immobilize, disintegrate, or inhibit metabolism of all life cycle stages
ex. ivermectin, albendazole
treating viral infections
viruses use our cellular pathways to replicate, so treatments often include inhibiting host cell pathways
vaccines are the best defense against viral infections
what does inhibition of viral cycles cause?
prevent viral entry, production of viral proteins/genome replication, virion maturation
how do we treat biofilms?
antimicrobials may not be able to penetrate the sticky layers of biofilms
when microbes in biofilm, they express different genes and have different susceptibility profiles
workarounds include treatment with additional molecules to enhance the effects of antimicrobials
how do microbes develop antimicrobial resistance?
they may acquire mutations or share genetic information
microbial mechanisms of drug resistance
synthesis of new enzymes which inactivate the drug
microbial cell doesn’t let drug in
drug is eliminated
binding sites for drug are deceased
microbe uses an alternate pathway
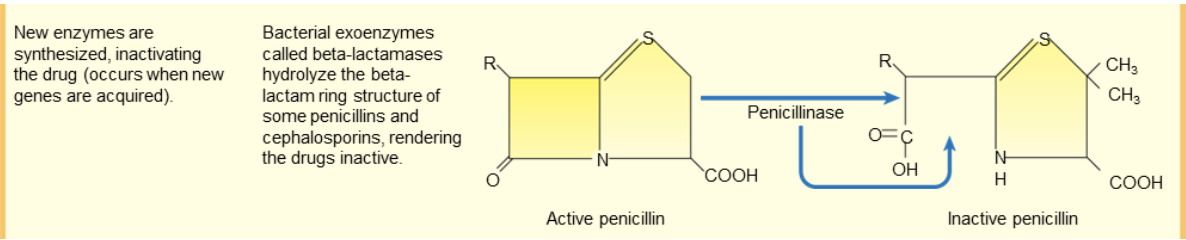
drug resistance: new enzyme synthesis
new enzymes synthesized which inactivate the drug
occurs when new genes are acquired
drug resistance: exclude drug from cell
permeability or uptake of the drug into the bacterium is decreased (occurs via mutation)

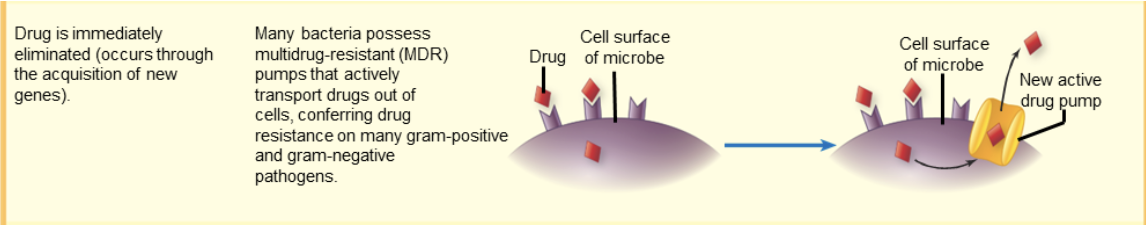
drug resistance: eliminate drug
drug immediately eliminated
occurs thru acquisition of new genes
many bacteria possess multidrug-resistant (MDR) pumps that actively transport drugs out of cells
drug resistance: binding sites decreased
binding sites for drugs are decreased in number and/or affinity
occurs via mutation or thru acquisition of new genes
erthromycin and clindamycin resistance associated with alteration on 50S ribosomal binding site

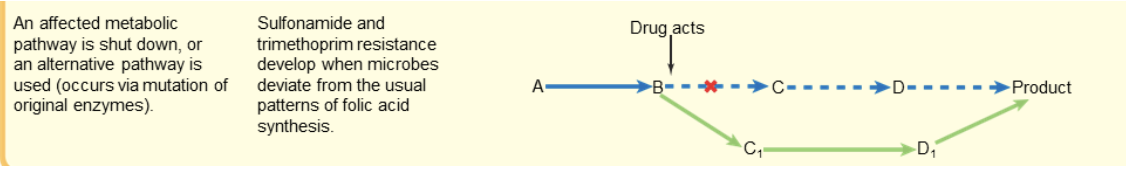
drug resistance: alternate pathways
an affected metabolic pathway is shut down
OR an alternate pathway is used
occurs via mutation of original enzymes
sulfonamide and trimethoprim resistance occurs when microbes deviate from the usual pattern of folic acid synthesis
how antibiotic resistance occurs?
high number of bacteria. few of them are resistant to antibiotics
antibiotics kill bacteria causing the illness as well as good bacteria protecting the body from infection
the resistant bacteria now have preferred conditions to grow and take over
bacteria can even transfer their drug-resistance to other bacteria, causing more problems
serious adverse reactions that are possible with beginning an antimicrobial treatment
toxicity to organs
allergic reactions
microbiome disruption
toxicity to organs
drug damages human organsall
allergic reactions
drug triggers the immune system
microbiome disruption
helpful microbes are killed
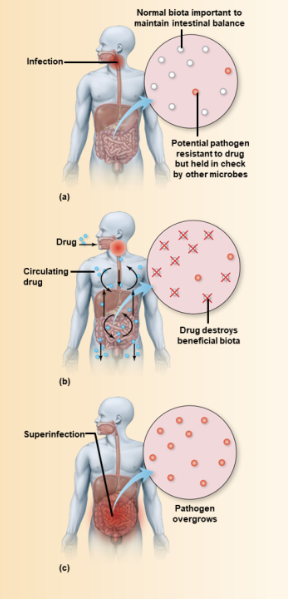
superinfection
microbiome disruption
after destruction, microbes which are usually present in small numbers overgrow and cause disease
ex. patient treated for UTI may develop yeast infection