IB BIO - U2L13 - Food as Fuel
1/33
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
34 Terms
What is the main organic compound in this process?
Glucose
What is ATP?
Adenosine Triphosphate.
High energy/Large amount of free energy molecule.
Universal energy currency.
What is ATP comprised of?
Adenine.
Ribose.
3 covalently bonded phosphate groups.
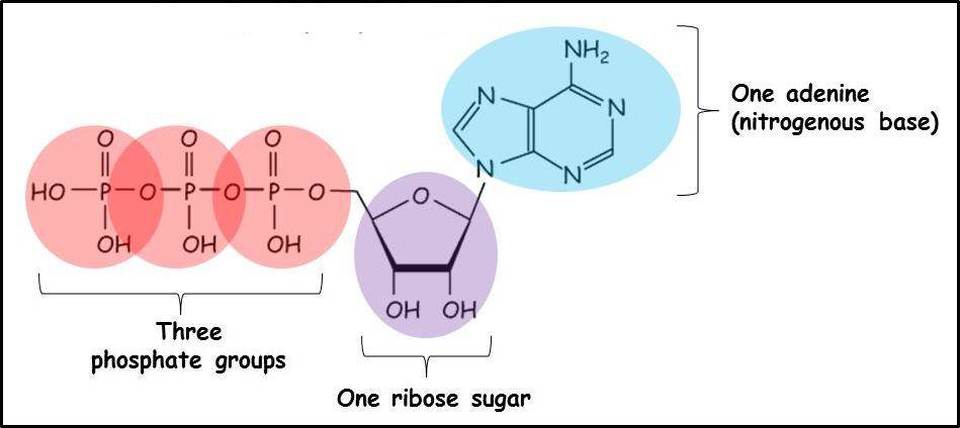
Cause of ATP’s free energy.
The high free energy of ATP is primarily attributed to the key pyrophosphate bond between its second and third phosphate groups. During hydrolysis, this bond is broken with the help of water, releasing energy. The energy release results from the increased stability of the hydrolysis products (ADP and inorganic phosphate, Pi) compared to the reactant ATP, providing the cell with usable energy for various processes.
ATP Hydrolysis.
ATP breaks down to produce ADP, a phosphate group and a large amount of energy.
ATP+H2O→ADP+Pi+energy
This reaction is catalyzed by enzymes known as ATPases (ATP synthase).
The energy is released because the products of the reaction (ADP and Pi) are more stable than ATP.
The released energy is often in the form of a phosphate group.
Exergonic (Releases Energy) Reaction.
Catabolic (Breakdown of Large to Small Molecules) Reaction.
ATP Synthesis.
Addition of a Phosphate to ADP.
Occurs through chemiosmosis most significantlly during oxidative phosphorylation specifically during ETC.
Requires Energy.
2 mechanisms by which this occurs.
Substrate-level Phosphorylation.
Oxidative Phosphorylation.
ADP+Pi+energy→ATP+H2O.
Condensation adds a phosphate to ADP.
Condensation reaction is where ADP and inorganic phosphate combine to form ATP releasing water.
This occurs through Substrate-level phosphorylation.
Anabolic (Synthesis of Small to Large molecules) reaction.
What is a Condensation reaction?
The joining of two molecules with the removal of a small molecule, often water.
What is Chemiosmosis?
Chemiosmosis is a biological process that involves the movement of ions across a selectively permeable membrane.
Particularly significant in cellular respiration and photosynthesis.
In what manner do ATP hydrolysis and ATP synthesis utilise water?
In ATP hydrolysis, water is added to the ATP molecule to break the high-energy phosphate bond between the second and third phosphate groups.
In ATP synthesis, water is released when ADP (adenosine diphosphate) and inorganic phosphate (Pi) react to form ATP.
What is ADP?
Adenosine diphosphate.
ATP Formation processes.
ATP can be synthesized in two ways.
Substrate level phosphorylation.
Oxidative phosphorylation.
Substrate level phosphorylation.
ATP formation via direct transfer of a high energy phosphate group from a substrate molecule to ADP.
Enzyme catalyzed (in that an enzyme is required) — occurs on enzyme surface.
Does not involve the electron transport chain or a proton gradient, unlike oxidative phosphorylation.
What is Substrate level phosphorylation characterised by?
Direct Transfer of Phosphate Group.
Occurs in the Cytoplasm.
Examples:
Glycolysis.
High-energy phosphate groups are transferred directly to ADP, forming ATP.
In Cytoplasm.
Citric Acid Cycle (Krebs Cycle).
In mitochondrial matrix.
Does Not Involve the Electron Transport Chain.
Energy.
ENERGY IS REQUIRED.
The energy comes from the chemical reactions that occur during the breakdown of high-energy substrate molecules.
Basically, energy released in spontaneous reactions.
Limited ATP Production.
Yield smaller relative to oxidative phosphorylation.
Typically associated with Catabolic reactions in that an intermediate phosphorylated metabolic molecule gets transferred directly onto ADP in a catabolic pathway converting it to ATP.
What is Oxidative phosphorylation (condensed)?
Oxidative phosphorylation is a process in cellular respiration where ATP is formed using energy indirectly transferred from a series of redox reactions along the electron transport chain. The involvement of oxygen as the final electron acceptor ensures the continuation of the electron flow, allowing cells to efficiently generate ATP for various energy-requiring processes.
What is Oxidative phosphorylation (expanded)?
Occurs during Cellular respiration, specifically in final stages of aerobic respiration.
Occurs in:
Inner mitochondrial membrane.
Eukaryotic cells.
ATP formation using energy indirectly transferred from a series of redox reactions.
Involves final electron acceptor.
Oxygen is electronegative.
Involves final electron acceptor.
In the ETC, electrons are passed through a series of protein complexes and energy released is used to actively pump protons (H⁺ ions) across the inner mitochondrial membrane.
Thus creating an electrochemical gradient, with a higher concentration of protons in the intermembrane space than in the mitochondrial matrix.
The final electron acceptor in the electron transport chain is molecular oxygen (O₂) in aerobic respiration.
Oxygen is highly electronegative, making it an excellent electron acceptor. Oxygen combines with electrons and protons to form water (H₂O), closing the electron transport chain.
What is Oxidative phosphorylation characterised by?
Catabolic:
Complex organic molecules, such as glucose, are broken down in a series of catabolic pathways to release energy. (This makes it an Exergonic reaction)
Aerobic:
Requires oxygen.
O₂ serves as the final electron acceptor in the electron transport chain (part of oxidative phosphorylation).
High electronegativity allows it to efficiently accept electrons and protons, forming water.
Energy Production:
ATP synthesis occurs through the phosphorylation of adenosine diphosphate (ADP) using the energy released during the transfer of electrons through the electron transport chain.
Electron Transport Chain:
Oxidative phosphorylation involves the (ETC).
Electrons from high-energy molecules (such as NADH and FADH₂) are transferred along the ETC, and the energy released is used to actively pump protons (H⁺ ions) across the membrane.
Chemiosmosis:
The proton gradient created by the active transport of protons across the membrane forms an electrochemical gradient.
This gradient is used in chemiosmosis, where protons flow back into the mitochondrial matrix (or the cell interior in prokaryotes) through ATP synthase.
ATP synthase utilizes this flow to phosphorylate ADP, forming ATP.
What is Cellular Respiration?
Complex set of metabolic pathways that cells use to extract energy from organic molecules, such as glucose.
Also the controlled breakdown of organic molecules over many steps.
Energy released captured via redox and used to generate ATP
The process involves multiple stages:
Glycolysis in the cytoplasm.
The Citric Acid Cycle (Krebs cycle).
In the mitochondrial matrix.
Oxidative phosphorylation in the inner mitochondrial membrane.
The ETC
What is a Redox reaction?
Short for reduction-oxidation reaction.
Chemical reaction that involving electron transfer between two substances.
One substance undergoes oxidation (loses electrons).
Undergoes reduction (gains electrons).
What is a characteristic feature of molecules that are considered high in energy?
Molecules with lots of C-H bonds are comparatively high in energy.
What process releases the potential energy stored in food molecules, particularly carbohydrates, during cellular respiration?
Potential energy stored in food molecules is released during oxidation reactions.
What is an Oxidation reaction?
Electron loss by a substance.
Characterized by an increase in the oxidation state or oxidation number of the reacting species.
Oxidation number can be determined via electron amount value which changes during oxidation reactions.
Within the context taught in class:
Oxidation:
Substance that undergoes oxidation is the reducing agent because it facilitates the reduction (gain of electrons) of another substance.
Electron Loss
Oxygen Addition.
Hydrogen removal
Reduction:
Substance that undergoes reduction is the oxidizing agent because it facilitates the oxidation (loss of electrons) of another substance.
Electron gain.
Oxygen removal.
Hydrogen addition.
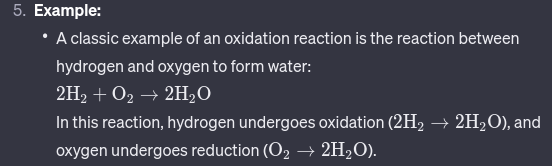
Mnemonic device for Oxidation Reactions.
LEO
Loss Electrons - Oxidization.
GER
Gain Electrons - Reduction.
Energy Carriers.
Energy carriers are molecules that temporarily store and transport energy within cells.
NADH
NAD+
What is NAD+ ?
Positively charged oxidized precursor carrier molecule.
By oxidized.
NAD+ is in the oxidized form and involves electron loss.
NAD+ being an electron carrier, involves accepting electrons from other molecules.
This acceptance of electrons results in the reduction of NAD+ to its reduced form, NADH.
Full name:
Nicotinamide adenine dinucleotide.
Two nucleotides joined by their phosphate groups.
Two nucleotides comprised of a nicotinamide base, an adenine base, and a ribose sugar.
High-end(energy) electron carrier. (Oxidized form)
NAD+ is a high-energy electron carrier.
Do we have to know why?
What is NADH?
Reduced form of NAD+.
Refer to flashcard on oxidation. (H addition)
Hydrogen carrier molecule.
Carries a pair of electrons and a proton temporarily.
Carries potential energy to drive ATP Synthesis.
The transfer of electrons from NADH, already in a high-energy state, to the electron transport chain in the inner mitochondrial membrane releases energy.
This energy is then used to actively pump protons (H+ ions) across the membrane, creating an electrochemical gradient.
NADH and NAD+ Relation (condensed).

Glucose Oxidation Reaction.
Formula to be memorised.
Glucose oxidation occurs through a series of enzyme catalyzed reactions.

What are the ways by which cells can extract energy from food in the absence of oxygen?
Anaerobic Respiration.
Fermentation.
What is Anaerobic Respiration?
Inorganic molecule, different from oxygen, serves as the final electron acceptor or oxidizing agent in the ETC to make ATP.
Occurs in Cytoplasm.
What is Fermentation?
Fermentation is an anaerobic metabolic process that allows cells to generate energy in the absence of oxygen to make ATP.
Fermentation relies on internal processes within the cell to regenerate electron carriers.
Types of fermentation.
Alcohol Fermentation
Lactate Fermentation.
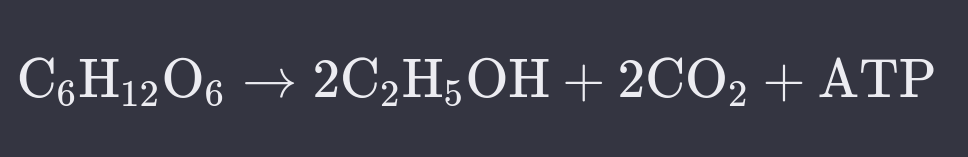
What is Alcohol Fermentation?
Glucose from food is converted to carbon dioxide, ethanol (alcohol), and ATP (energy).
Examples:
Bread making.
Beer brewing.
Wine.
Employed by plants and yeasts with no oxygen.
Yeast employs alcohol fermentation solely as it is prokaryotic with no mitochondria.
What is Lactate Fermentation?
Glucose, from food, is converted to lactate and ATP (energy).
Examples.
Yoghurt.
Some cheeses.
Strenuous Excercise.
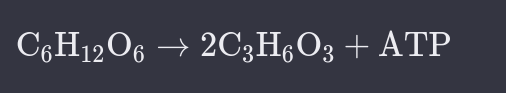
How do cellular respiration pathways utilize electron transport systems to generate ATP through oxidative phosphorylation?
Respiration pathways use electron transport systems to generate ATP by oxidative phosphorylation.
What distinguishes fermentation pathways from other cellular processes, particularly in terms of their absence of electron transport systems and the lack of ATP synthesis through oxidative phosphorylation?
Fermentation pathways lack electron transport synthesis.