Topic 6: Acids, Bases, and Buffers - The pH Scale
1/33
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
34 Terms
pH - Definition
Concentration of H+ ions relative to that in pure water to identify the acidic/basic nature of a system
pOH - Definition
Concentration of OH- ions relative to that in pure water to identify the acidic/basic nature of a system
pH Scale and its Mechanism
Adding acid to water → [H₃O⁺] ↑ and [OH⁻] ↓ to maintain the Kw constant by reforming products into reactants
Adding base to water → [OH⁻] ↑ and [H₃O⁺] ↓ to maintain the Kw constant by reforming products into reactants.
Acid/base addition can shift concentrations over 14 orders of magnitude.
H₂O + H₂O ⇌ H₃O⁺ + OH⁻.
![<ul><li><p>Adding <strong>acid</strong> to water → [H₃O⁺] ↑ and [OH⁻] ↓ to maintain the Kw constant by reforming products into reactants</p></li><li><p>Adding <strong>base</strong> to water → [OH⁻] ↑ and [H₃O⁺] ↓ to maintain the Kw constant by reforming products into reactants.</p></li><li><p>Acid/base addition can shift concentrations over <strong>14 orders of magnitude</strong>.</p></li><li><p>H₂O + H₂O ⇌ H₃O⁺ + OH⁻.</p></li></ul><p></p>](https://knowt-user-attachments.s3.amazonaws.com/62c62086-08d5-4a1a-8d7b-d47359881a7a.png)
pH - Formula
pH = –log₁₀[H₃O⁺]
Change of 1 in the pH = change in [H₃O⁺] by a factor of 10
pOH - Formula
pOH = –log₁₀[OH⁻].
Change of 1 in the pH = change in [OH⁻] by a factor of 10
Relationship Between pH and pOH
pH + pOH = 14 (at 25 °C).
Calculating [H₃O⁺] and [OH⁻] from pH and pOH
[H₃O⁺] = 10⁻ᵖᴴ
[OH⁻] = 10⁻ᵖᴼᴴ.
pH Scale - Neutral
pH = 7 ([H₃O⁺] = [OH⁻])
![<p>pH = 7 ([H₃O⁺] = [OH⁻])</p><p></p>](https://knowt-user-attachments.s3.amazonaws.com/ce868647-a087-4d3a-9b86-3d8f08594dd4.png)
pH Scale - Acidic
pH < 7 ([H₃O⁺] > [OH⁻]).
![<p>pH < 7 ([H₃O⁺] > [OH⁻]).</p><p></p>](https://knowt-user-attachments.s3.amazonaws.com/789efcfe-ae28-44ac-a18b-ad24cdcce8ec.png)
pH Scale - Basic
pH > 7 ([H₃O⁺] < [OH⁻]).
![<p>pH > 7 ([H₃O⁺] < [OH⁻]).</p><p></p>](https://knowt-user-attachments.s3.amazonaws.com/66522946-96e1-426d-8094-4d75b50e3160.png)
Ionization
Neutral molecular compounds (usually covalent) react in water to form ions that weren't there before - proton transfer reaction
E.g: HCl (g) + H₂O (l) → H₃O⁺ (aq) + Cl⁻ (aq).
Dissociation
Ionic compound already has ions in its lattice. When dissolved, the ions separate into solution.
E.g: NaCl (s) → Na⁺ (aq) + Cl⁻ (aq).
Whenever dissociation occurs, the real acid-base reaction referred to for the calculations is the ionization undergone later by the product that will act as an acid/base
NH₄Cl (s) → NH₄⁺ (aq) + Cl⁻ (aq)
The acid-base chemistry centers around NH₄⁺ as the Cl⁻ is just a spectator ion. NH₄Cl is just a solution of NH₄⁺
Strong Acids
Most are covalent molecules (e.g. HCl, HNO₃, H₂SO₄).
In water, they ionize completely (not dissociate, because they are not ionic solids to begin with).
Have very weak conjugate bases as a result, leaving the conjugate base and H+ in the solution
Reaction: HCl + H₂O → H₃O⁺ + Cl⁻
Arrow: Single arrow (→), because ionization is essentially 100%.
It can be assumed that [H₃O⁺] are stoichiometrically equal to the concentration of the acid in question, because it ionizes in one step
Strong Acids to Remember
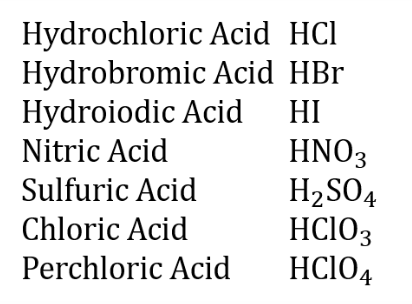
Weak Acids
Also covalent molecules (e.g. CH₃COOH, HF).
In water, they ionize partially (proton transfer to water, equilibrium between ionized and unionized form).
Reaction: CH₃COOH + H₂O ⇌ H₃O⁺ + CH₃COO⁻
Arrow: Double equilibrium arrow (⇌), because ionization is incomplete.
Some weak acids can have multiple conjugate bases from how many H+ ions it loses from each deprotonation step
E.g: Citric acid (H₃C₆H₅O₇) → 1st: H₃C₆H₅O₇ ⇌ H⁺ + H₂C₆H₅O₇⁻ → 2nd: H₂C₆H₅O₇⁻ ⇌ H⁺ + HC₆H₅O₇²⁻ → 3rd: HC₆H₅O₇²⁻ ⇌ H⁺ + C₆H₅O₇³⁻
Strong Bases
Many are ionic compounds (e.g. NaOH, KOH, Ca(OH)₂).
In water, they simply dissociate completely into pre-existing ions.
Reaction: NaOH (s) → Na⁺ (aq) + OH⁻ (aq)
Arrow: Single arrow (→), since dissociation is essentially 100%.
It can be assumed that [OH-] are stoichiometrically equal to the concentration of the base in question, because it dissociates in one step
Strong Bases to Remember

Weak Bases
Typically molecular compounds (e.g. NH₃, amines).
They ionize in water by proton transfer (accept H⁺ from H₂O to form OH⁻).
Reaction: NH₃ + H₂O ⇌ NH₄⁺ + OH⁻
Arrow: Double equilibrium arrow (⇌), because only partial ionization occurs.
Acid Dissociation Constant - Ka
Smaller Ka → weaker acid; larger Ka → stronger acid.
Smaller pKa → stronger acid; larger pKa → weaker acid.
Where aA + bB ⇌ dD + eE
Ka = [D]d[E]e/[A]a[B]b
pKa = -log10Ka
Units: mol/L
Base Dissociation Constant - Kb
Smaller Kb → weaker base; larger Kb → stronger base.
Smaller pKb → stronger base; larger pKb → weaker base.
Where aA + bB ⇌ dD + eE
Kb = [D]d[E]e/[A]a[B]b
pKb = -log10Ka
Conjugate Pairs - Strong and Weak Acids and Bases
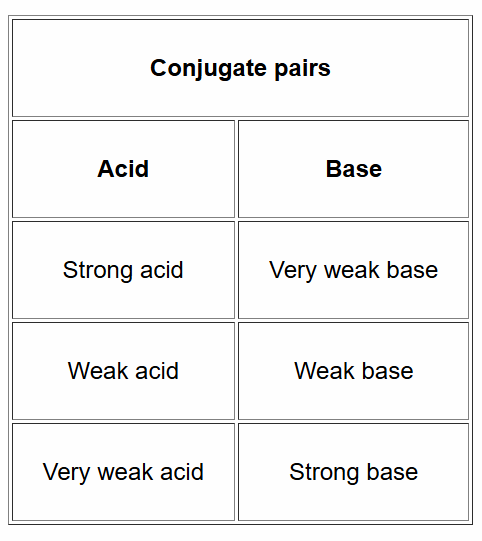
Ka, Kb, and Kw Relationships
For a conjugate acid–base pair: Ka × Kb = Kw = 1.0 × 10⁻¹⁴.
Therefore: pKa + pKb = 14 (at 25 °C).
Weak Acid/Base Pairs
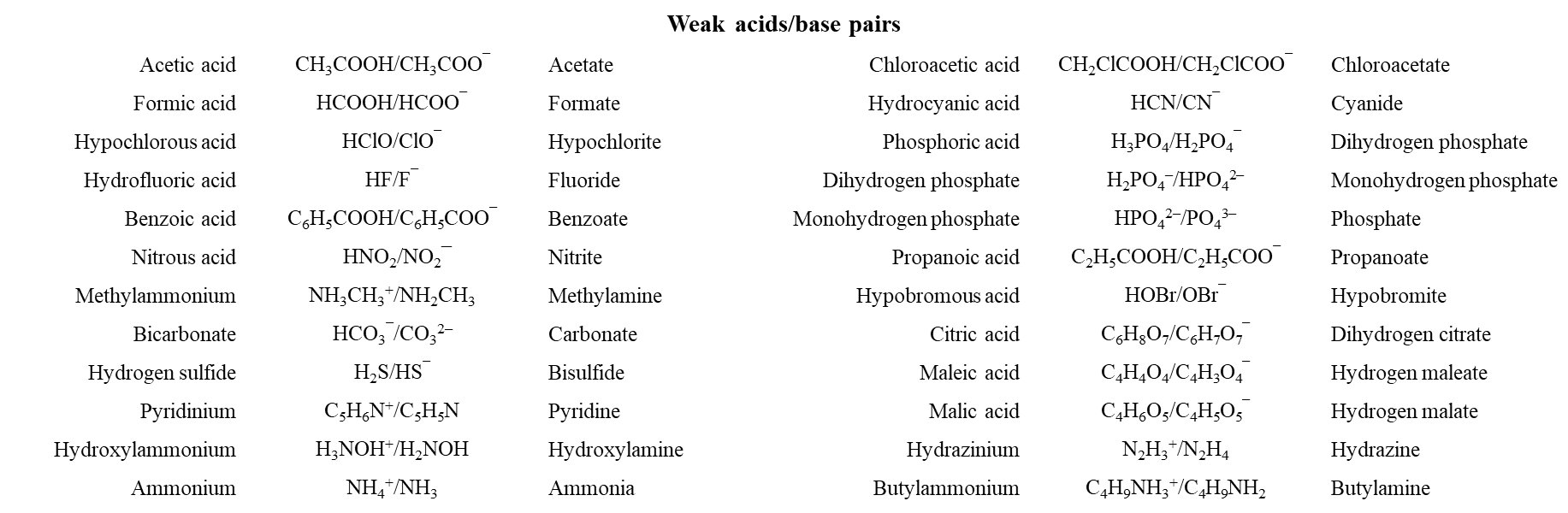
pH Calculations
Goal: Find the [H₃O⁺] or [OH⁻] to be plugged into the pH or pOH formula. Set up an ICE table to find the concentrations of the substances involved, then sub them into the Ka/Kb formula to find the [H₃O⁺] or [OH⁻]. Note that since Ka or Kb is so small, x is negligible.
Strong acids: [H₃O⁺] ≈ initial acid concentration.
Strong bases: [OH⁻] ≈ initial base concentration.
Weak acids/bases: [H₃O⁺] or [OH⁻] must be calculated using Ka or Kb.
Use Kw to interconvert: [H₃O⁺] × [OH⁻] = 10⁻¹⁴.
pH + pOH = 14.
Common Polyatomic Ions
Cations
Ammonium: NH₄⁺
(Most others are anions)
Anions
Hydroxide: OH⁻
Nitrate: NO₃⁻
Nitrite: NO₂⁻
Carbonate: CO₃²⁻
Bicarbonate (hydrogen carbonate): HCO₃⁻
Sulfate: SO₄²⁻
Sulfite: SO₃²⁻
Hydrogen sulfate (bisulfate): HSO₄⁻
Phosphate: PO₄³⁻
Hydrogen phosphate: HPO₄²⁻
Dihydrogen phosphate: H₂PO₄⁻
Acetate: CH₃COO⁻ (sometimes written C₂H₃O₂⁻)
Cyanide: CN⁻
Permanganate: MnO₄⁻
Chromate: CrO₄²⁻
Dichromate: Cr₂O₇²⁻
Ionic Compound - Metal + Nonmetal
Electrons transferred from metal → nonmetal.
Forms a crystalline lattice of cations + anions.
Example: NaCl, MgO, CaF₂.
Ionic Compound - Metal + Polyatomic ion
Still ionic (metal forms cation, polyatomic ion is an anion).
Example: NaNO₃, CaSO₄.
Ionic Compound - Polyatomic Cation + Polyatomic Anion
Less common but exists.
Example: NH₄NO₃ (ammonium nitrate).
Molecular (Covalent) Compound - Nonmetal + Nonmetal
Electrons shared, molecules formed.
Example: H₂O, CO₂, CH₄.
Tip on Weak Acids
If a problem provides a pKa value, it’s a weak acid.
General Acid Dissociation Reaction
HA (aq) + H₂O (l) ⇌ H₃O⁺ (aq) + A⁻ (aq)
Ka = [H₃O⁺][A⁻]/[HA]
Water is the solvent and its concentration is considered constant, so it is omitted from the equilibrium expression.
Weak Acid Dissociation Reaction
HA ⇌ H⁺ + A⁻
At equilibrium, [H⁺] ≈ [A⁻], and the initial acid concentration [HA]₀ is much greater than the amount dissociated
Ka ≈ [H⁺][A-]/ [HA]
Ka vs. pKa and Kb vs. pKb Relationship
Each 10-fold increase in Ka corresponds to a 1-unit decrease in pKa.
Each 10-fold increase in Kb corresponds to a 1-unit decrease in pKb.
And vice versa
General Base Dissociation Reaction
B (aq) + H₂O (l) ⇌ BH⁺ (aq) + OH⁻ (aq)
[BH⁺][OH⁻]/[B]