13 Halogenoalkanes complete Saga
0.0(0)
0.0(0)
New
Card Sorting
1/12
Study Analytics
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
13 Terms
1
New cards
Halogenoalkanes contain ?
polar bonds
2
New cards
Why do Halogenoalkanes contain polar bonds ?
as the halogens are more electronegative than carbon atoms
3
New cards
In halogen bond is C-X which is x (s+ or s-) (Halogen)
X = s-
4
New cards
Nucleophiles species are ?
'positive liking'
5
New cards
Nucleophiles contain a lone electron pair that is attracted to ?
∂+ / S+ regions of molecules
6
New cards
Common Nucleophiles ?
● CN: -
● :NH3
● - :OH
● :NH3
● - :OH
7
New cards
What must nucleophiles be shown with ?
e lone electron pair and often a negative sign
8
New cards
Nucleophilic Substitution - reaction mechanism that shows how nucleophiles attack ?
halogenoalkanes
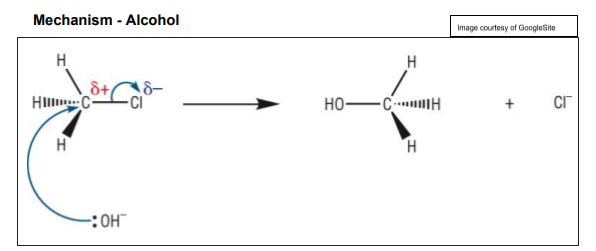
9
New cards
The greater the Mr of the halogen in the polar bond, the ?
lower the bond enthalpy
10
New cards
Nucleophilic substitution reactions can only occur for 1 o (primary) and ?
2o (secondary) halogenoalkanes
11
New cards
When a halogenoalkane is heated to high temperatures under alcoholic conditions What occurs ?
elimination occurs
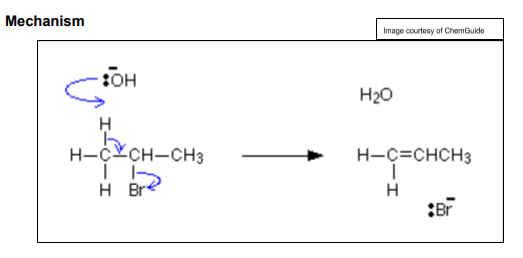
12
New cards
Elimination reactions can only occur from what type of halogenoalkanes ?
2 o and 3o (tertiary)
13
New cards
Ozone in the atmosphere absorbs ?
UV radiation.