ihs 340 exam 2 content
1/92
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
93 Terms
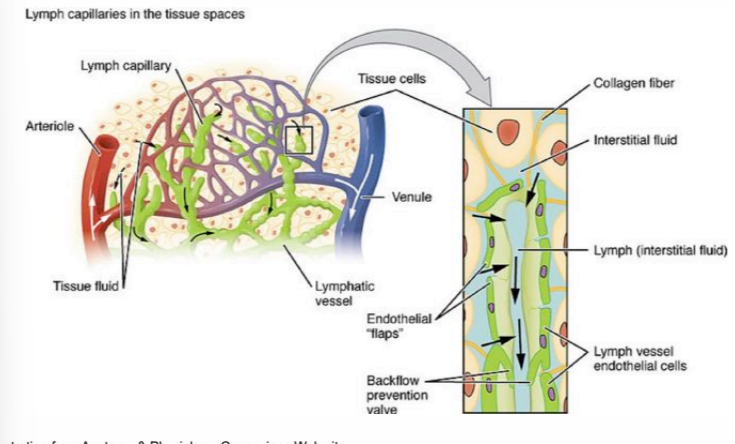
how does lymph leave the lymphatic system?
it moves through one-way valves that open with fluid pressure
IL
interleukins
chemokine nomenclature
CCL#, CXCL#, CX3CL# and they bind to receptors that are named CCR#, CXCR#, and CX3CR#
CD
cluster of differentiation, cell surface molecules
five cardinal signs of inflammation
redness, warmth, pain, swelling, altered function
“inflammation”
can be acute or chronic
characterized by redness, warmth, pain, swelling, and altered function in external tissues or joints OR leukocyte infiltrates into a tissue, increased vascular permeability, and enhanced cytokine production in the tissue
when do monocytes differentiate into macrophages?
after being released into the blood
macrophages in the body
every tissue in the body has macrophages. . . they keep the place clean!
neutrophils
most abundant white blood cell in the body
granulocyte, aka polymorphonuclear leukocytes
“ambulance” of the immune system
typically predominant cell in pus
why are neutrophils considered the ambulance of the immune system?
produced in high numbers quickly in bone marrow upon infection and travel to site of infection to exert anti-microbial activity
phagocytosis
the process by which a cell uses its plasma membrane to engulf a large particle, giving rise to an internal compartment called the phagosome
which cells carry out phagocytosis?
primarily macrophages and neutrophils
what is needed for phagocytosis to occur?
phagocytes need to recognize the surface of a particle as foreign
opsonin
an antibody or other substance that helps mediate phagocytosis
opsonization
the process by which opsonins coat microorganisms and bind to receptors on phagocytes to improve chance of phagocytosis
some phagocytosis receptors are opsonin-independent
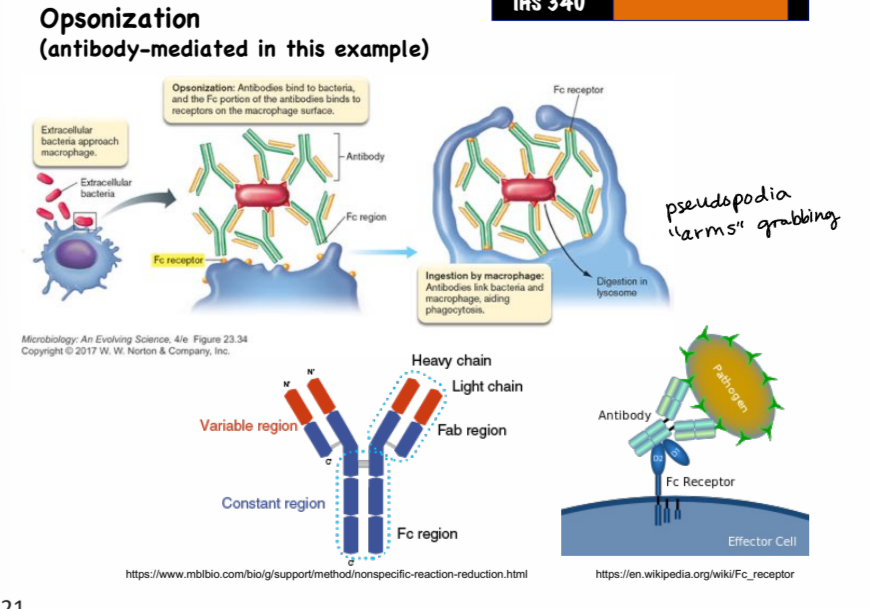
what are some examples of opsonins?
antibodies and C3b
what is the “don’t eat me” signal?
expression of cell-surface glycoprotein CD47 by host cell, it signals to phagocytes to not eat the cell even if bound by an opsonin or via opsonin-independent pathways
steps in phagocytosis
bacterium binds to the surface of phagocytic cells, antibodies or complement (opsonins) can aid binding
phagocyte pseudopods extend and engulf the organism
invagination of phagocyte membrane traps the organism within a phagosome
a lysosome fuses and deposits enzymes into the phagosome, enzymes cleave macromolecules and generate reactive oxygen species, destroying the organism
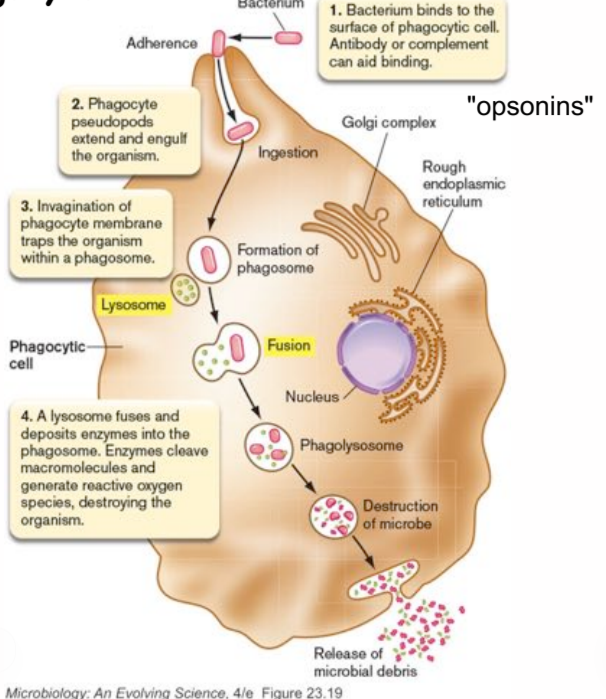
how do phagocytic cells kill ingested bacterial cells?
using reactive oxygen radicals
respiratory burst
a series of catalyzed reactions in the phagolysosome that creates reactive oxygen species, in the phagocytic vacuole of the phagocyte
phagolysosome
phagosome fused with a lysosome, degradative enzymes and reactive oxygen species found here
netosis by neutrophils
neutrophils “throw” NETs (neutrophil extracellular traps) around pathogens
NETosis is an unusual form of apoptosis, where neutrophil dies and releases a latticework of DNA imbued with AMPs into the surrounding area
complement
first discovered as a heat-liable component of blood that enhances the killing effect of antibodies on bacteria
complement source and composition
mainly from liver
made of ~20 proteins naturally present in serum
some are proteases that form and cleave other complement factors
how do complement proteins work?
they are formed in the liver and circulate through the system via blood as inactive precursors, they interact with each other to form an activation cascade
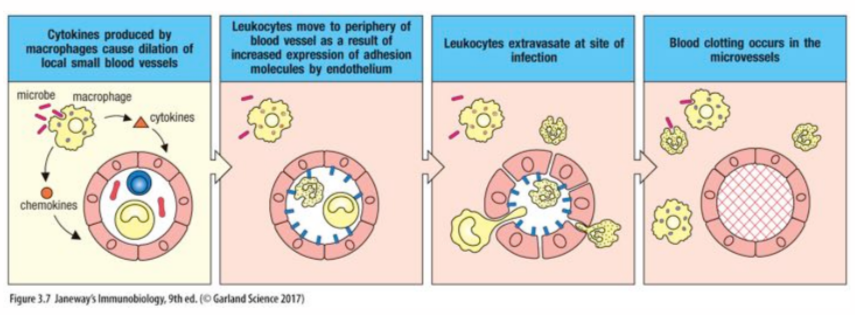
chemokines
class of cytokines that signal for leukocyte migration (chemotaxis) to the injury site
functions by creating a chemotaxis gradient (high around producing cell and decreasing levels as you move away) that leukocytes move along
cytokines TNF-alpha, IFN-gamma, IL-1 and IL-4
stimuli that induce endothelial cell adhesion molecule expression
cytokines IL-6, C3a, C4a, C5a, and histamine
signal to endothelial cells to promote endothelium (vascular) barrier leak
sialyl lewis^X
a tetrasaccharide carbohydrate usually attached to O-glycans on the surface of cells
expressed on granulocytes and monocytes, and mediates inflammatory extravasation (fluid leakage) of these cells
not present in resting B and T cells but strongly expressed upon activation
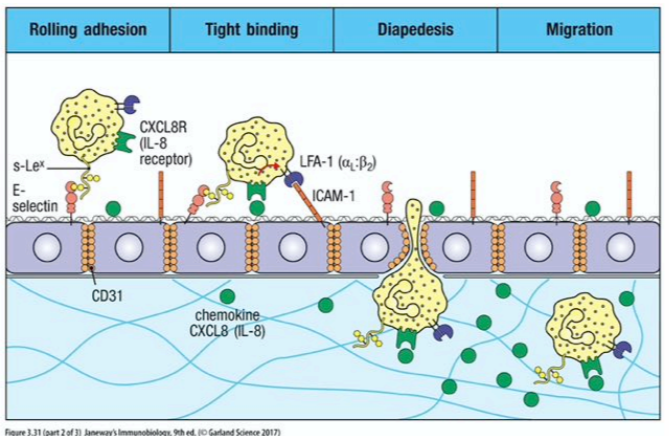
migration of leukocytes into an inflamed or infected tissue
leukocytes roll along the vascular endothelial surface, between adhesion to sialyl-lewis^X groups is weak
neutrophils cleave through epithelial cell tight junctions to exit the vessel to get to site of infection
why didn’t initial immune therapies work?
because we didn’t understand the redundancies in the system and how it functions as a whole
cell-adhesion molecules role in inflammatory response
they control interactions between leukocytes and endothelial cells during the inflammatory response
examples: ICAM, VCAM, PECAM
cell-surface bound and function as both ligands and receptors
innate immunity response to damage
tissue resident cells detect cellular/tissue damage and microorganisms, induce an inflammatory response to contain the infection by producing cytokines and other mediators
degenerate receptors
receptors that bind to multiple structurally similar ligands, or receptors that bind to multiple ligands that exhibit similar surface charge
affinity of degenerate receptors for their various ligands will likely be different and may affect the generation of secondary messengers
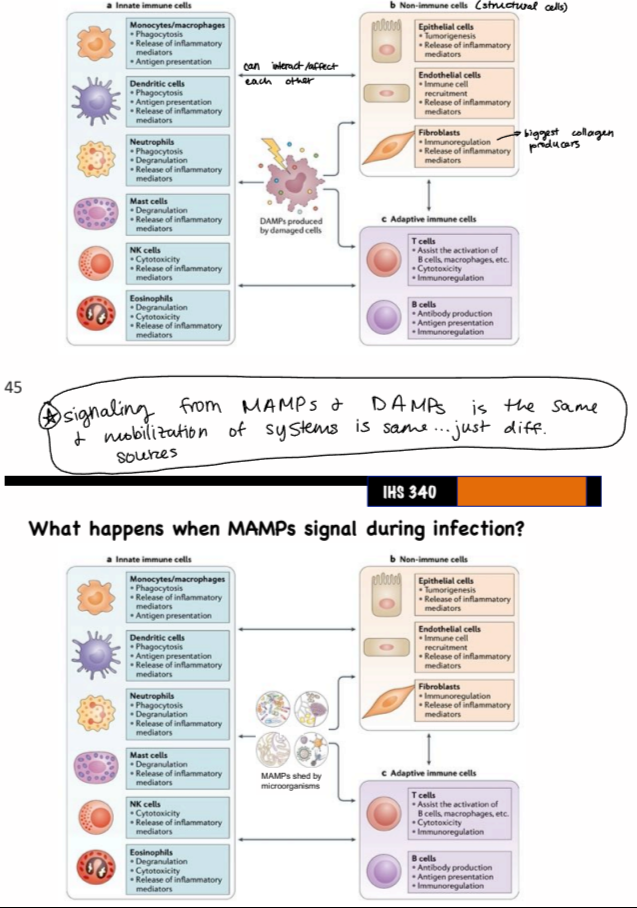
MAMPs
small molecular structures that are conserved within a class of microorganisms and recognized by degenerate receptors of the innate immune system
examples: lipopolysaccharides, teichoic acids, dsRNA, CpG dinucleotides, flagellin
DAMPs
molecules released upon cellular stress or tissue injury
endogenous danger signals, because they induce potent inflammatory responses by activating the innate immune system during non-infectious cellular/tissue damage
can originate from extracellular, intracellular, and plasma proteins
PRRs
host-encoded degenerate receptors that bind MAMPs and DAMPs
expressed on phagocytic WBCs and many other cells in the body
found on external surface of cell and inside cell
examples: TLRs, NLRs, etc.
result of signaling from MAMPs and DAMPs
same mobilization of cells and systems (innate and adaptive immune cells, non-immune cells), just different sources (cell damage vs. microorganisms)
formation of atherosclerotic plaque
macrophages are eating lipid droplets in blood vessel, and it can accumulate and block the vessel
mediated by scavenger receptors which are degenerate receptors
efferocytosis
process by which apoptotic cells are removed by phagocytic cells
mammalian toll-like receptors (TLRs) are activated by. . .
many different MAMPs
cellular locations of mammalian TLRs
transmembrane proteins
some are on cell surface and detect extracellular MAMPs
some are located intracellularly in walls of endosomes, can recognize MAMPs only accessible after microorganism has been broken down after phagocytosis
after binding to ligand, all TLRs dimerize, forming either heterodimers or homodimers
endosomes
vesicles created for endocytosis, receptor-mediated process
what can TLR signaling activate?
transcription factor NFkB, which drives expression of pro-inflammatory cytokines
initial genetic rearrangements in a B cell to create a novel antibody
D and J segments are recombined, then V segment is recombined to DJ segment
after transcription, splicing, translation, and assembly, a new antibody is made with a unique variable region
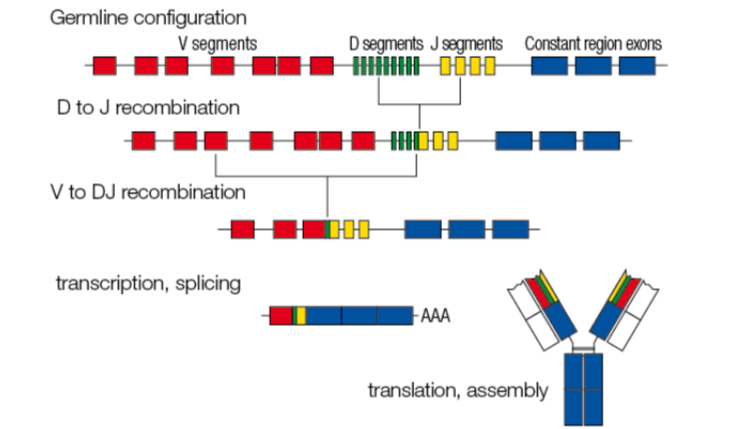
junctional diversity
recombination of genetic blocks (V, D, J regions) can be a few nucleotides off, resulting in frameshifts of the codons used for selecting amino acids for heavy and light chain proteins
frameshifts create new codons, which ultimately generate more distinct antibody possibilities
how many possible novel antibody combinations can be made by B cells in the body?
5.2 × 10^13
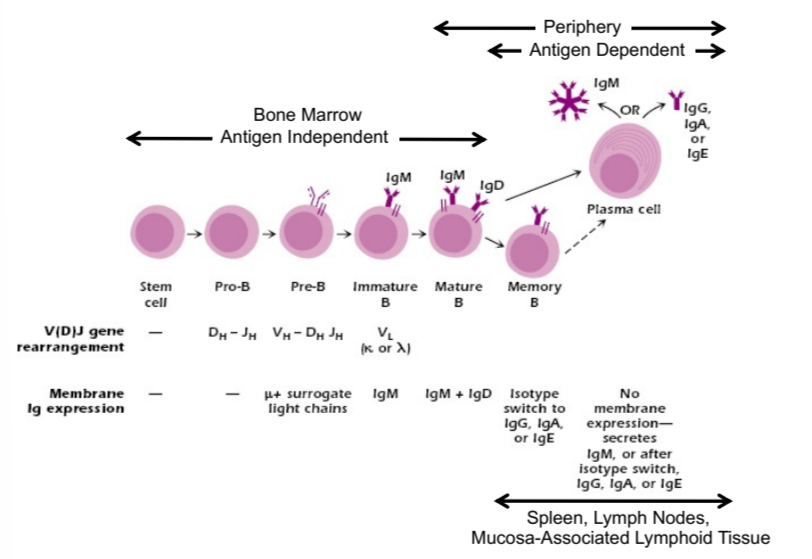
B cell development
in bone marrow:
antigen independent
from stem cell to proB to preB to immature B to mature B to memory B and plasma cells
antibodies are formed during this stage, mature cells expressing IgM and IgD
in periphery:
antigen dependent
expresses IgM or IgG, A, or E
B cell clonal selection and differentiation in the periphery
antigen-dependent process
if no ligand bound within a certain amount of time, B cell undergoes apoptosis
a bound antibody on a B cell triggers clone proliferation, stronger signal elicits more proliferation
antigen receptor specificity generation and ramifications
initial specificities of the antigen receptors are generated by genetic rearrangement and high degree of mutation
receptors can have unpredicted specificity, like to allergens and self antigens
to control this, secondary signals are needed to activate cells by antigen receptors, like co-stimulatory signals
cytokine storm
an uncontrolled and excessive release of cytokines into the system, makes you feel sick, cytokines end up acting like hormones
T cell receptor molecule (TcR) structure
surface-bound antigen receptor on T cells, similar to antibody but doesn’t circulate in the blood
monovalent heterodimer (alpha/beta or gamma/delta chains, with alpha/beta predominating)
generated by recombination of V, D, J regions in TcR genetic loci, NOT Ig loci
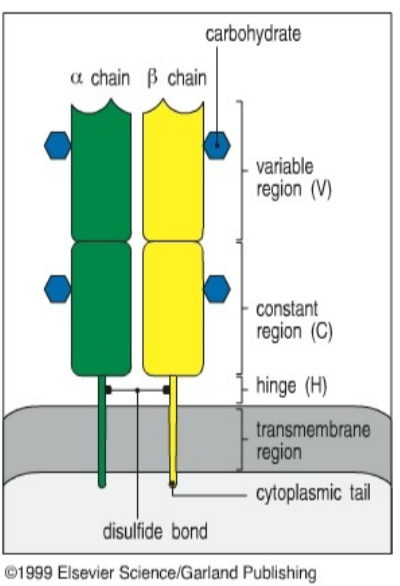
potential diversity of TcRs
about 10^18 possible combinations
T cell development
they start in bone marrow but mature in the thymus
major difference between Ig and TcR
how they bind antigens. . .
antibodies bind directly to Ag at the epitope, while TcRs bind to small fragment of Ag (peptide) when it’s helped in place by MHC molecule, a fragment that’s typically not on the surface of the non-denatured antigen
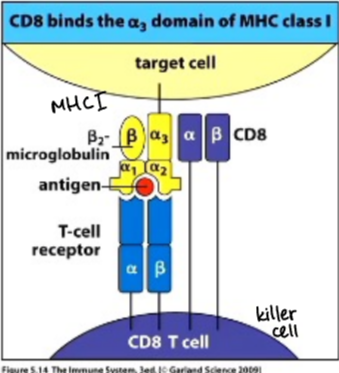
major histocompatibility complex (MHC) molecules
act as identification molecule for cells, function is to hold short AA stretch (peptide) from antigen
degenerate receptors, binding to multiple types of peptide fragments
explain the TcR-MHC interaction
MHC receptor on antigen-presenting cell is holding Ag peptide in peptide binding groove, and TcR on T cell binds to Ag peptide fragment
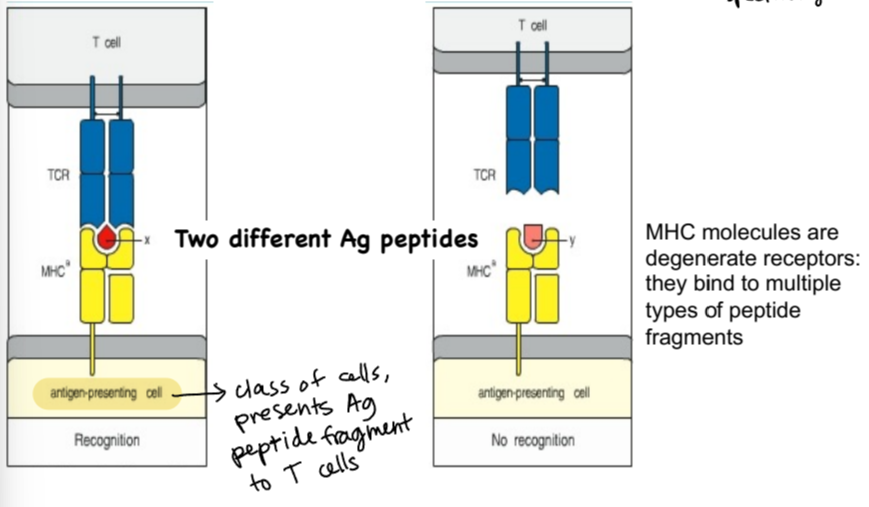
MHC expression
MHC I - found on all nucleated cells in the body, acting as an ID card to other cells
MHC II - found on antigen-presenting cells (APCs) at high levels at baseline and can go even higher in the presence of inflammation and cytokines
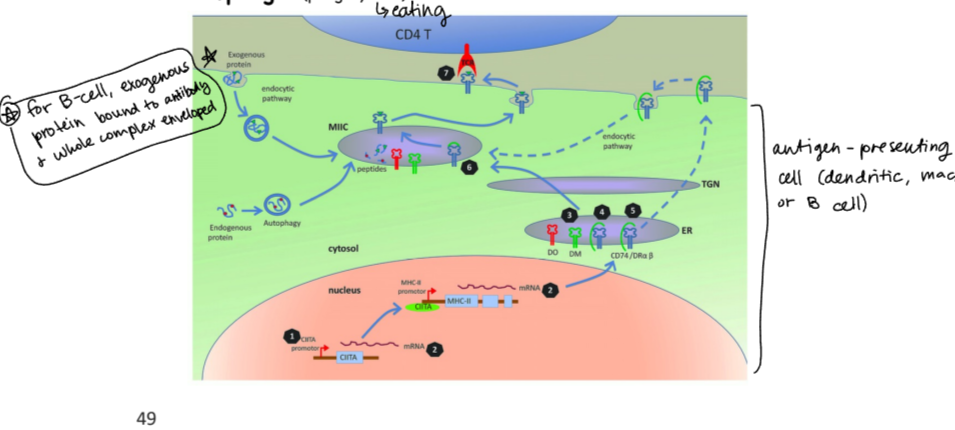
MHC II-expressing antigen-presenting cells
dendritic cells, macrophages, B cells
CD4 T cells
interact with cells that express MHC II, helper T cells (Th) because they provide activating signals for cells of the immune system
CD8 T cells
interact with cells expressing MHC I, in general are involved in killing virus-infected cells, also called cytotoxic T cells (CTL or Tc)
antigen presenting process by cells expressing MHCII
exogenous protein is enveloped (along with Ag if B cell)
MHC II promoter causes expression of various proteins, which then combine with endogenous and exogenous peptides to make MHC II receptor
receptor is embedded in membrane so it can interact with TcR
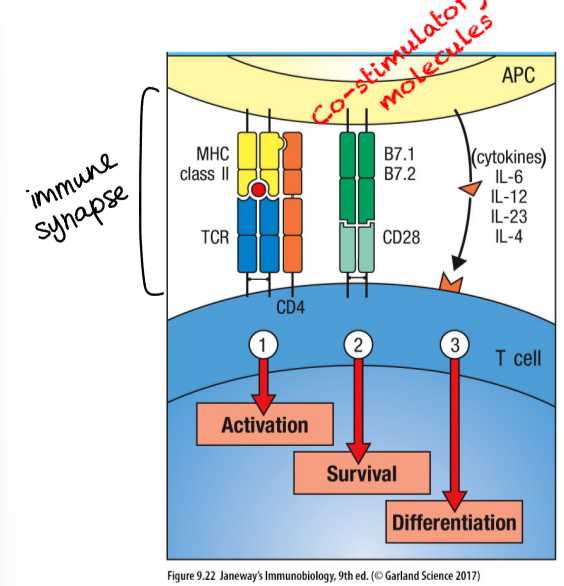
three kinds of signals for clonal expansion and differentiation of naive T cells
activation
survival
both of these result in T cell proliferation (clonal expansion)
differentiation - results in generation of effector T cells, that undergo additional changes that distinguish them functionally from naive T cells
T cell - B cell reciprocal interaction
B cell is antigen-presenting cell that mobilizes T cell, and CD4 T cell targets B cells to mobilize them with differentiation signals
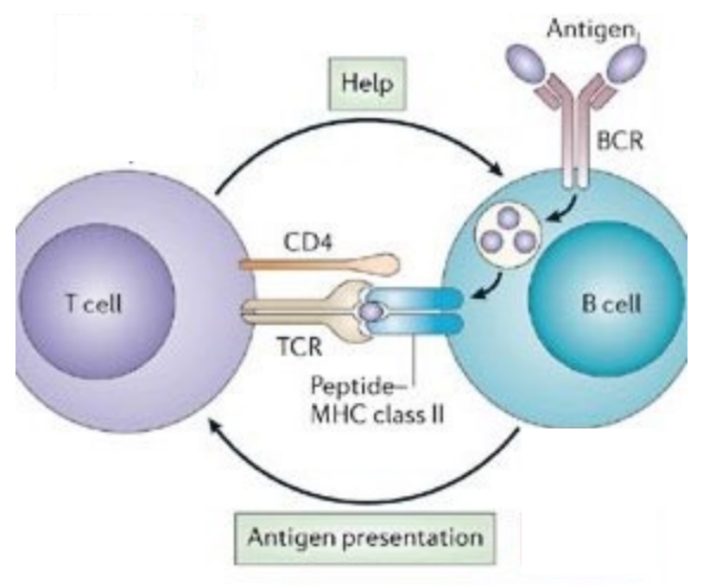
where do APC - T cell - B cell interactions occur?
the spleen and lymph nodes
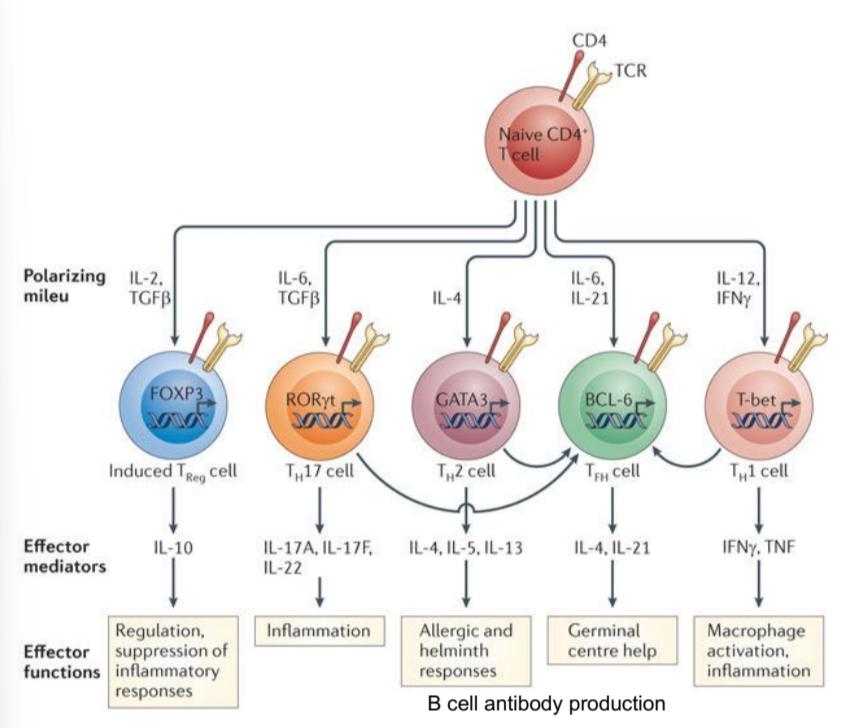
diverse roles of T cells in regulating immunity
naive T cells are exposed to polarizing mileu (ILs, TGFbeta, IFNgamma) that create subsets of effector T cells, which then release effector mediators/cytokines to cause functions like inflammation, allergic responses, etc.
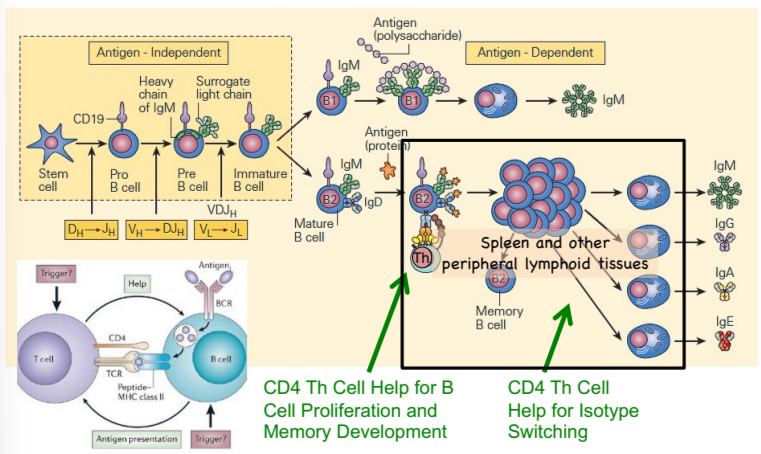
Th - B cell interactions in the spleen and other peripheral lymphoid tissues
CD4 Th cells also help B cells switch antibody isotype (IgM to IgA, etc.)
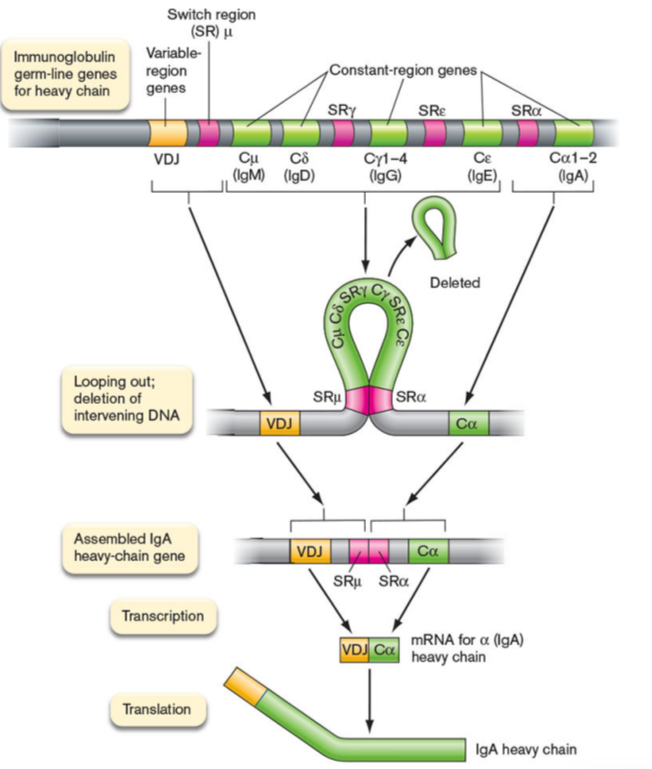
isotype switching
gene rearrangement of the constant region genes in heavy chain locus
Ag specificity of Ig remains the same but the structural end, aka Fc region, changes
which cells drive B cell development, including proliferation and isotype switching?
CD4 Th cells
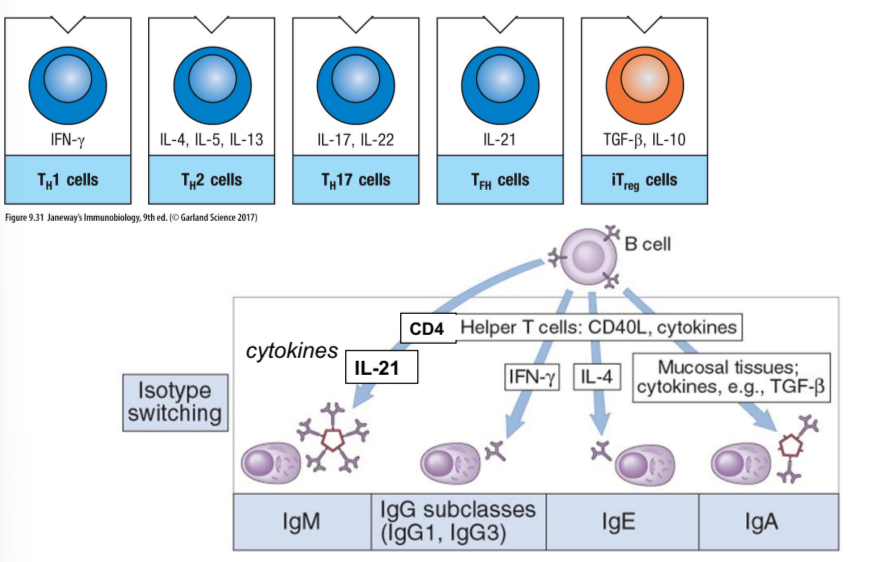
why would a B cell undergo isotype switching?
the function of an antibody is due to its heavy chain
B cells switch their heavy chains when they get a specific Th cell signal that also reacts to the same antigen
antibody isotype labels what happens next to the antigen
Fc receptor
cell surface receptor that binds to Fc region of an antibody
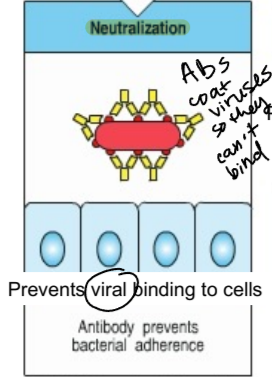
neutralization
process in which antibodies coat viruses and bacteria so they can’t bind to cells
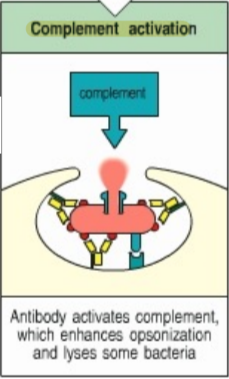
complement activation
antibody activates a complement, which enhances opsonization and lyses some bacteria
which cells/molecules are involved in immunity against parasites and also allergic responses?
B cells, Th cells, IgE, and mast cells
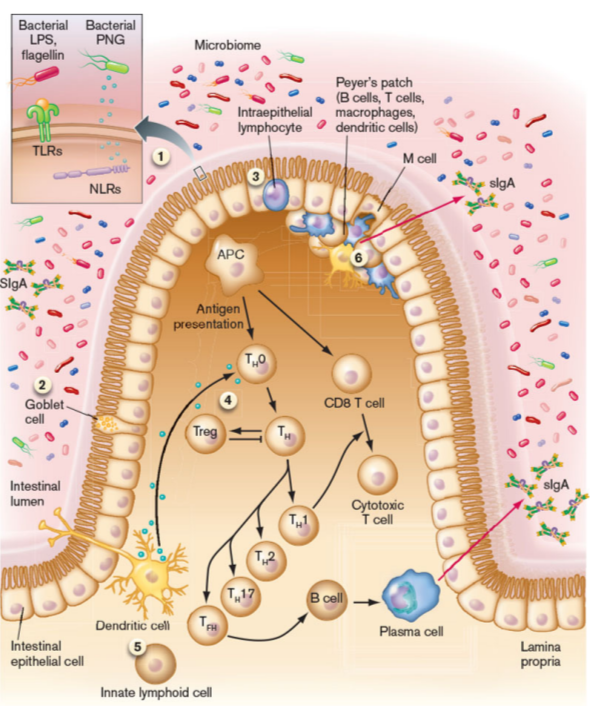
sIgA
made in the intestines in large quantities
major function is to bind to bacteria microbiota and prevent it from invading the mucus layer and adhering to epithelium, without inducing inflammation (mucosal tolerance)
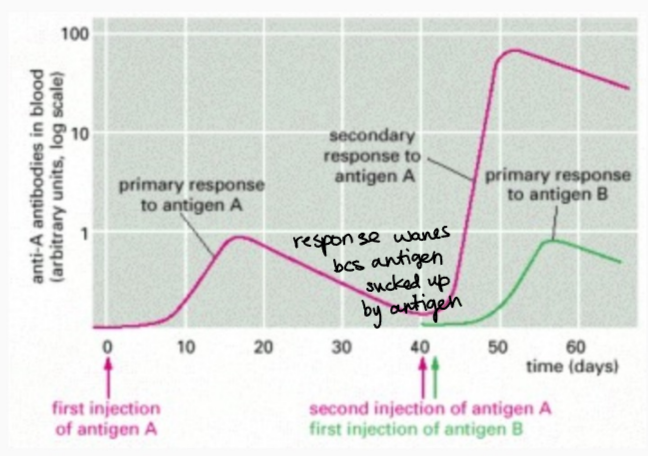
mechanism of booster vaccine or secondary Ag exposure
a secondary exposure greatly increases the response speed and level of antibodies compared to the primary response. . . also a new isotype of the antibody emerges
what keeps B cells from making antibodies that bind to host antigens (auto-antibody)?
mechanisms of antigen-reactive elimination of cells, aka MAGEC
occurs in bone marrow and in periphery, another line of immune system regulation
doesn’t work for aeroallergens and food because they are environmental antigens!
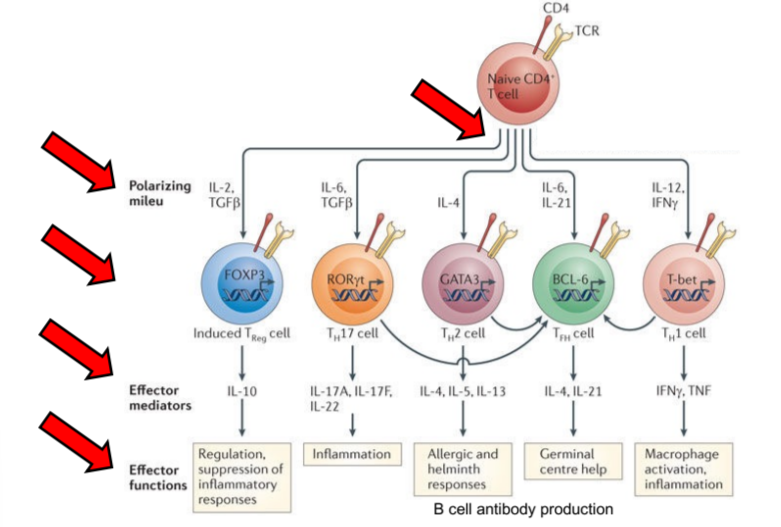
key control points for allergic diseases in development of Th cells
polarizing milieu, induced Th cells, effector mediators, effector functions
what is infection and disease after exposure determined by?
route of transmission, length of exposure, dose of inoculum, route of acquisition, type of infectious agent, and level of pre-existing immunity
how do we change the level of immunity in an individual?
vaccines!
what are vaccines?
a manipulation of adaptive immune system in an antigen-specific manner to mimic infection by a specific pathogen
results in stimulation of protective immunity against a pathogen without causing the disease of that pathogen
history of variolation
the transfer of a superficial skin would or inhalation of material from smallpox pustules, can protect or kill individual
practiced since 1400s in Middle East and China
edward jenner
developed vaccine to prevent disease, was familiar with variolation and used it to inoculate a child with cowpox material (milder than smallpox)
variola virus
variola major - most common and problematic viral form of smallpox
variola minor - smallpox viral variant causing fewer systemic symptoms, less extensive and fewer fatalities
linear dsDNA virus in same family as cowpox and monkeypox
types of immunizations
active and passive (like mother to child, temporary)
types of vaccine immunity
individual and herd
herd immunity
indirect protection of susceptible individual from infectious disease that happens when a population is immune either through vaccination or through previous infection
stops or slows the spread of an infectious disease as a result of there being less susceptible individuals available or immune individuals shedding less virus for a shorter period of time when infected
can be herd immunity against disease (visible) and herd immunity against infection (invisible)
percentage of people who need to be immune in order to achieve herd immunity varies with each disease
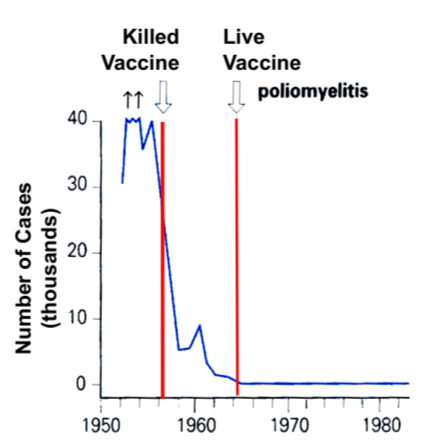
vaccine effectiveness in polio
the dead vaccine decreased the number of cases drastically, but the later development of the live vaccine decreased cases to essentially 0
immunologic adjuvant
any substance acting to accelerate, prolong, or enhance antigen-specific immune responses when used in combination with specific vaccine antigens. . .optimizes immune response to injected antigen
usually a combination of oil and water, like a micelle
can be synthetic or obtained naturally
what are some benefits of adjuvants?
decreasing dose of antigen needed
decrease number of vaccine doses needed
enhance vaccine efficacy in at-risk individuals
improve rapid and long-lasting immune responses
induce robust cell-mediated immunity
provide broad protection (cross-reactivity)
types of modern vaccines
attenuated microbes, “live vaccine” like the smallpox vaccine
killed microorganisms
subcellular microbial fragments or toxins (like HepB or tetanus)
microorganism DNA in a harmless virus as a delivery construct
microorganism RNA in a liposome as a delivery construct
mRNA vaccines have greatest potential and fewest drawbacks so far!
mRNA modification for vaccines
ends are modified and also the mRNA is delivered in a lipid coat, all to make it more stable
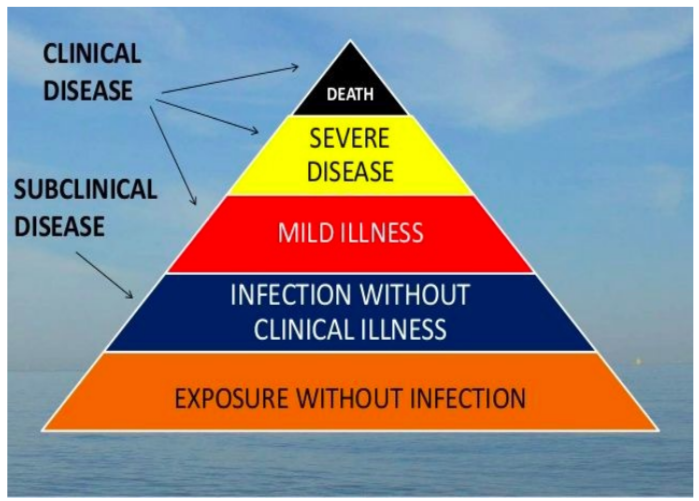
public policy question: which categories of the clinical disease pyramid are the most important output regarding vaccine effectiveness?
Dr. H —> output of vaccines should concern disease, decreasing intensity of symptoms and not necessarily preventing infection completely