Chapter 6 Reaction Summary
1/75
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
76 Terms
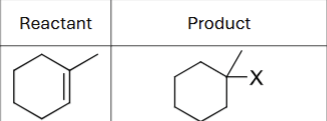
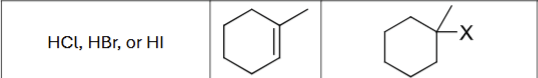
What are the possible reagents?
HCL, HBr, or HI

What is the product?
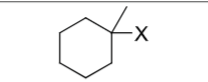
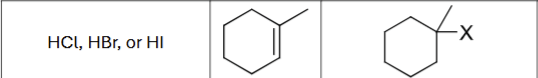
Does Markovnikov’s rule apply?
Yes
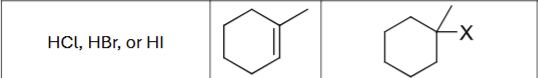
Are there possible carbocation rearrangements?
Yes
Possible stereochemistry?
No
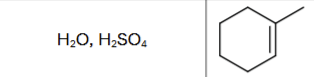
What is the product?
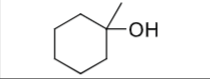
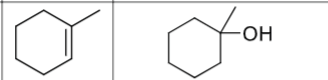
What are the reagents?
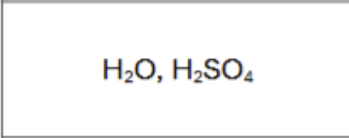

Does Markovnikov’s Rule Apply?
Yes

Is there any stereochemistry in this reaction?
No

Could there be carbocation rearrangement?
Yes
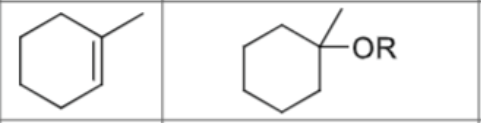
What are the reagents?
What is the product?


Does this reaction follow Markovnikov’s rule?
Yes

Does this reaction have carbocation rearrangements?
Yes

Does this reaction have any stereochemistry?
No
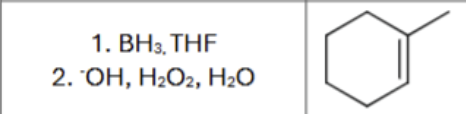
What is the product?
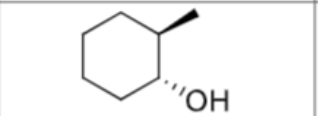
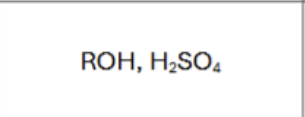
What are the reagents?
does Markovnikov’s rule apply?
No, Anti Markovnikov occurs
Any stereochemistry?
Yes,
Syn Addition
Are there any carbocation rearrangements?
No

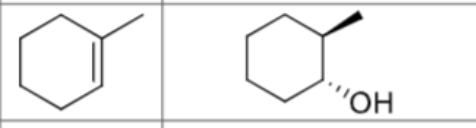
What is the product?
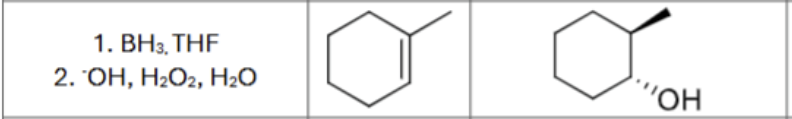
What are the reagents?


Is there any regiochemistry/does markovnikov apply?
No

Is there any stereochemistry?
Anti-Addition
Trans stereochemistry

Can there be carbocation rearrangements?
No
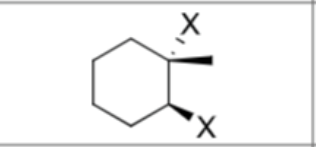
What are the reagents?

Does Markovnikov apply?
Yes
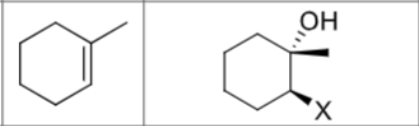
Is there any stereochemistry?
Yes,
Anti Addition
trans
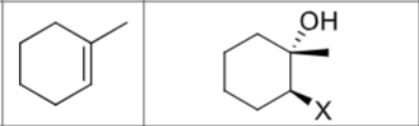
Can there be carbocation rearrangements?
No

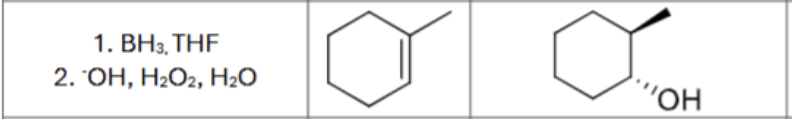
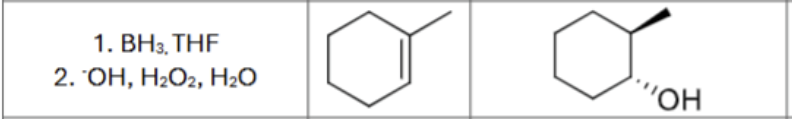
What is the product?
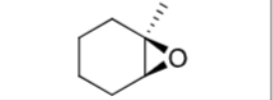
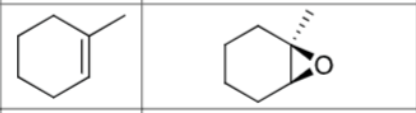
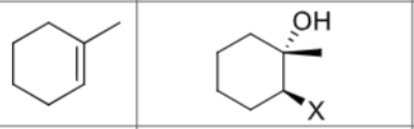
What are the reagents?
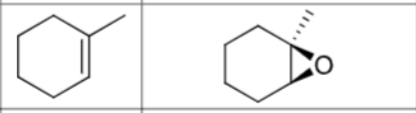
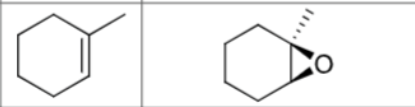
Does Markovnikov’s rule apply?
No
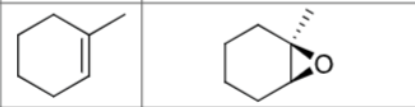
Is there any stereochemistry?
Yes,
Syn-Addition
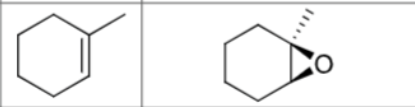
Are there carbocation arrangements?
No

What is the product?
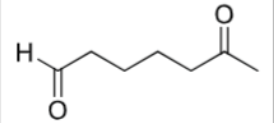
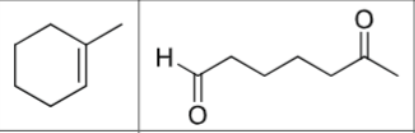
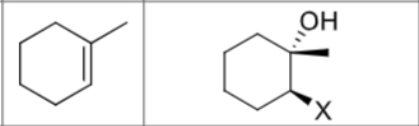
What are the reagents?
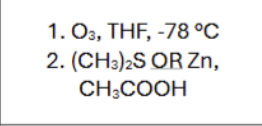
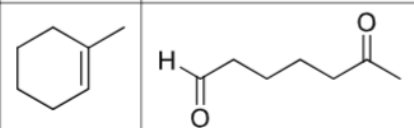
Is there any regiochemistry?
No
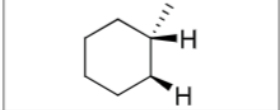
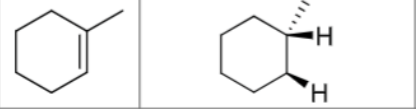
Is there any stereochemistry?
No
Can there be carbocation rearrangements?
No

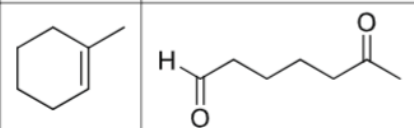
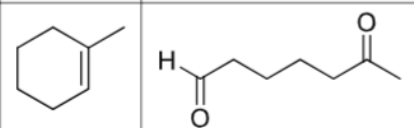
what is the product?
What are the reagents?
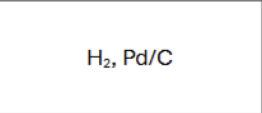
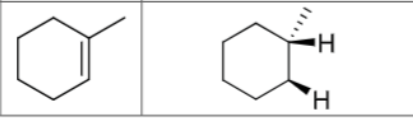
Is there any regiochemistry?
No
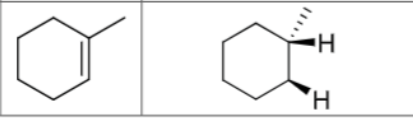
Is there any stereochemistry?
Yes
Syn Addition
Cis
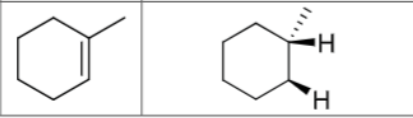
Can there be carbocation rearrangements?
No

Product?
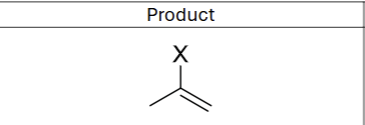

Is there any regiochemistry?
Yes, Markovnikov (X goes to more substituted Carbon)

Is there any stereochemistry?
No

What does excess reagent form?
geminal dihalide
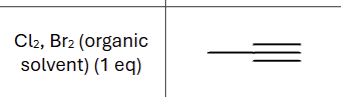
Product?
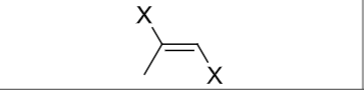

Is there any regiochemistry?
No

Any stereochemistry?
Anti-addition;trans halogenation

What does excess reagent form?
tetrahalide
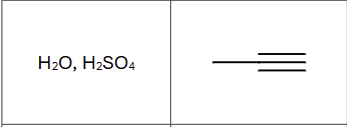
Product?
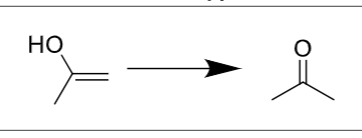
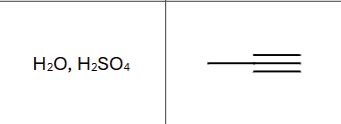
Is there any regiochemistry?
Markovnikov, OH to more substituted carbon
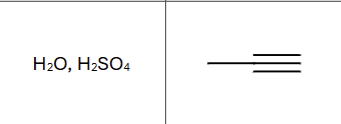
Is there any stereochemistry?
No, tautomerizes immediately
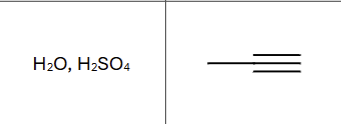
What is the final product (compound type/class)
ketone
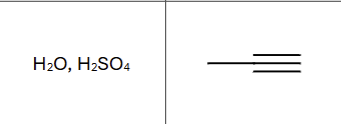
If a terminal alkene is used, what does it require?
Mercury catalyst
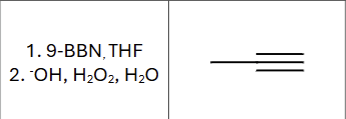
Product?
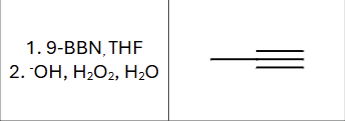
Any regiochemistry?
Anti-markovnikov (OH goes to less substituted Carbon)
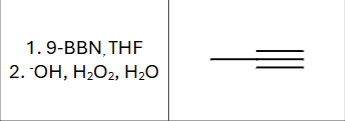
Is there any stereochemistry?
No, the product immediately tautomerizes
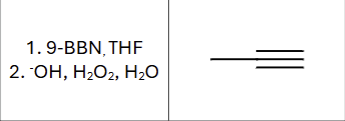
What is the compound type produced?
ketone or aldehyde

What is the product?
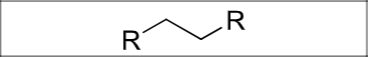

Is there any regiochemistry or stereochemistry?
No
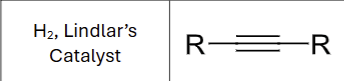
Product?
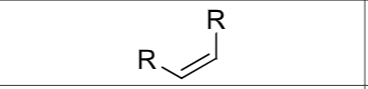
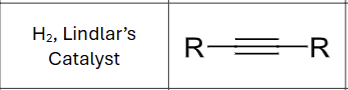
What stereochemistry exists?
Cis hydrogenation

Product?
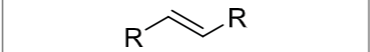

What stereochemistry exists?
Trans hydrogenation
Addition of reagents that form a carbocation intermediate have what type of stereochemical addition?
syn and anti
When reagents form a carbocation intermediate, how many stereoisomers are formed?
cis and trans isomers
What type of reactions have syn addition?
Addition of H2, Addition of a peroxyacid, Addition of BH3 and BHR2
Addition of Br2, Br2 and Water, Br2 and ROH all have ____ addition
anti
If an alkyne is asymmetrical, how many geminal dihalides will it form when reacted with a hydrogen halide?
2
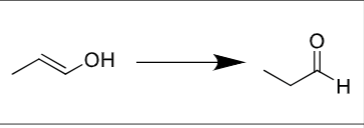
Enols and ketones are ______; constitutional isomers in equilibrium
tautomers
Water added to a asymmetrical alkyne will create…
2 ketones
In hydroboration-oxidation (BrH2), internal alkynes will form…
ketones
In hydroboration-oxidation (BrH2), terminal alkynes will form…
aldehydes