UNT Principles of Biochemistry Exam Two Review: Chapter 8 Flashcards
1/21
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
22 Terms
Lipid
Class of naturally occurring organic compounds classified based on common solubility properties. Amphipathic in nature
*insoluble in water, soluble in diethyl ether, chloroform, and acetone
Lipid Forms
Open Chain Forms: fatty acids, tricylglycerols, sphingolipids, phosphoacyglycerols, glycolipids
-lipid-soluble vitamins
-prostaglandins, leukotrienes, and thromboxanes
Cyclic Forms: cholesterol, steroid hormones, and bile acids
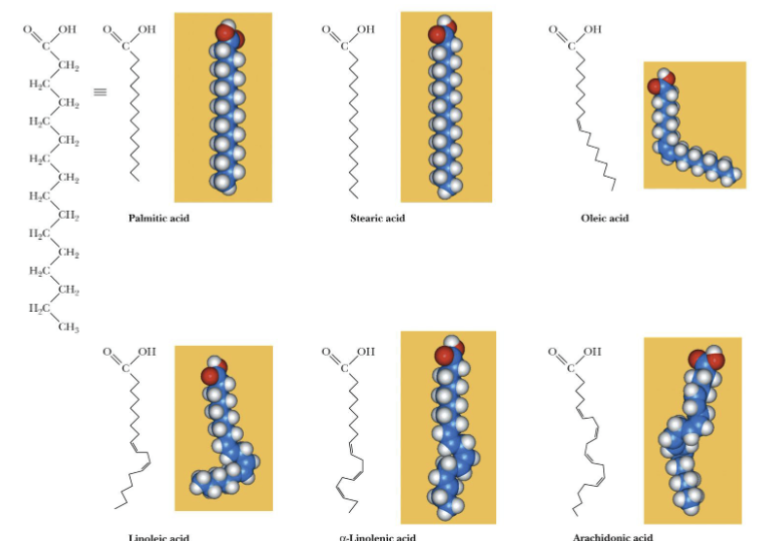
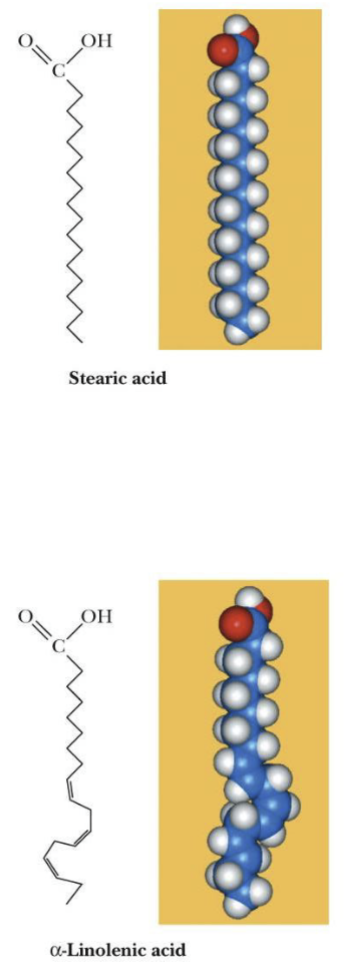
Fatty acid
Unbranched-chain carboxylic acid, most commonly 12-20 carbons, the double bonds and # of carbons are represented as two numbers and separated with a colon (18:0)
*cis isomer predominates, trans isomer is rare
Fatty acids that have C=C are unsaturated, C-C are saturated
Unsaturated Fatty Acids
Have lower melting points than saturated fatty acids
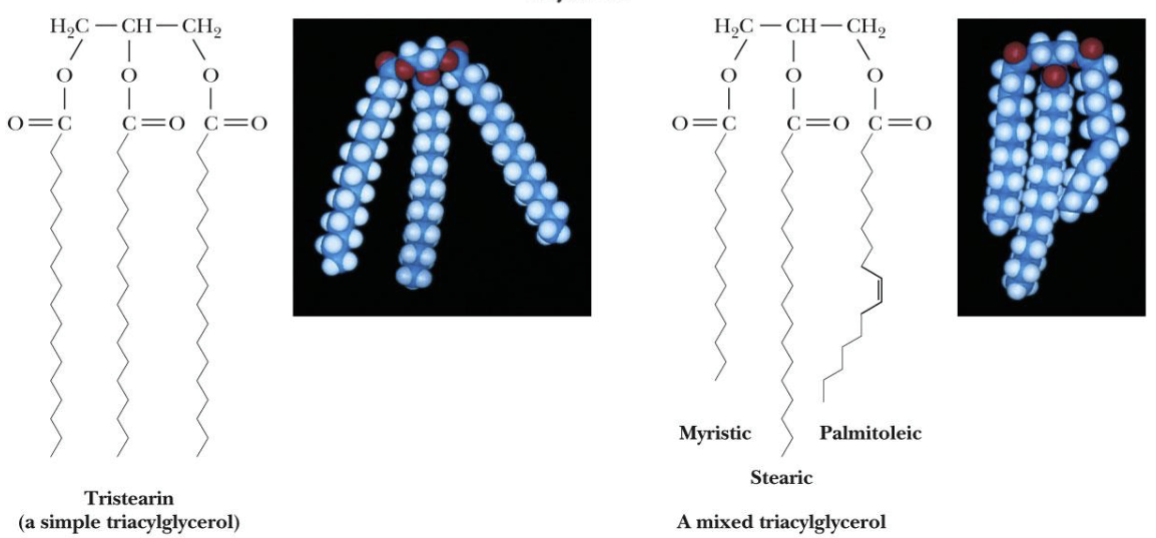
Triacylglycerol (triglyceride)
Ester of glycerol with 3 fatty acids
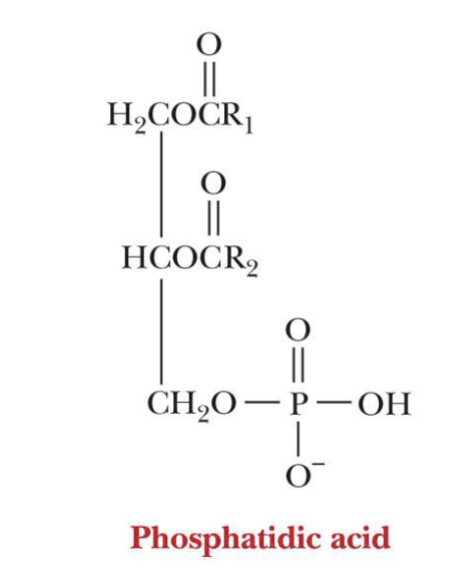
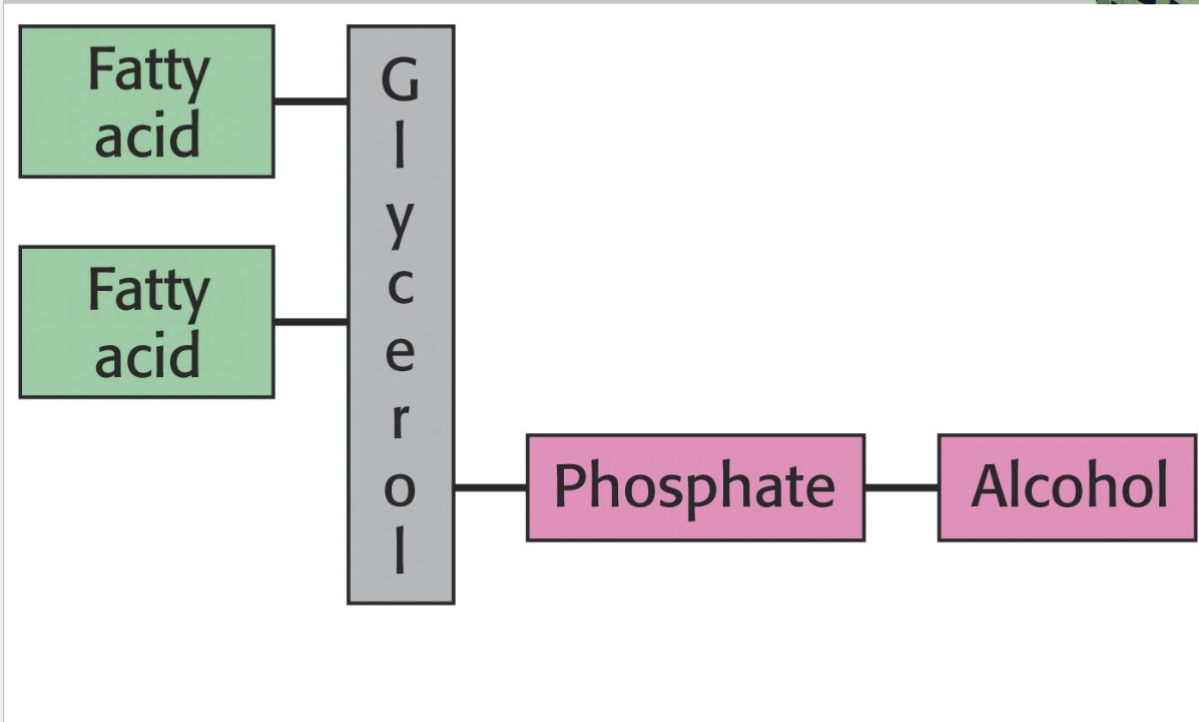
Phosphoacylglycerols (Phospholipids)
One alcohol group of phosphoric acid ?
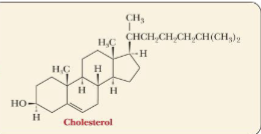
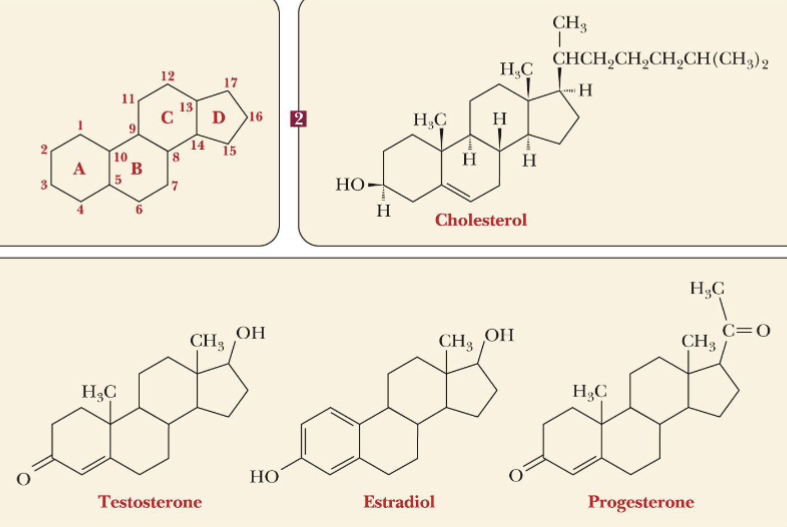
Steroids
group of lipids that have a fused-ring structure of 3 six-membered rings and 1 five-membered ring
Biological Membranes*
In aqueous solution, phosphoglycerides spontaneously
form into a lipid bilayer, with a back-to-back arrangement
of lipid monolayers
• polar head groups are in contact with the aqueous
environment
• nonpolar tails are buried within the bilayer
• the major force driving the formation of lipid bilayers
is hydrophobic interaction
• the arrangement of hydrocarbon tails in the interior
can be rigid (if rich in saturated fatty acids) or fluid (if
rich in unsaturated fatty acids)
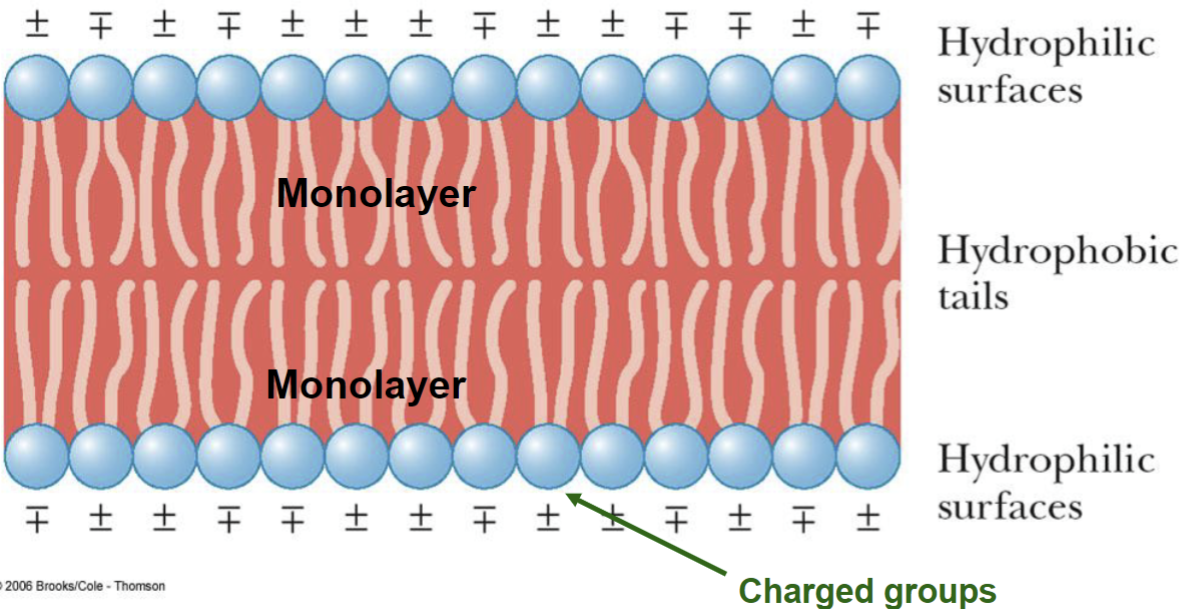
Lipid Bilayers*
Schematic drawing of portion of a bilayer consisting of phospholipids
the polar surface of the bilayer contain charge groups
the hydrocarbon “tails” lie in the interior of the bilayer
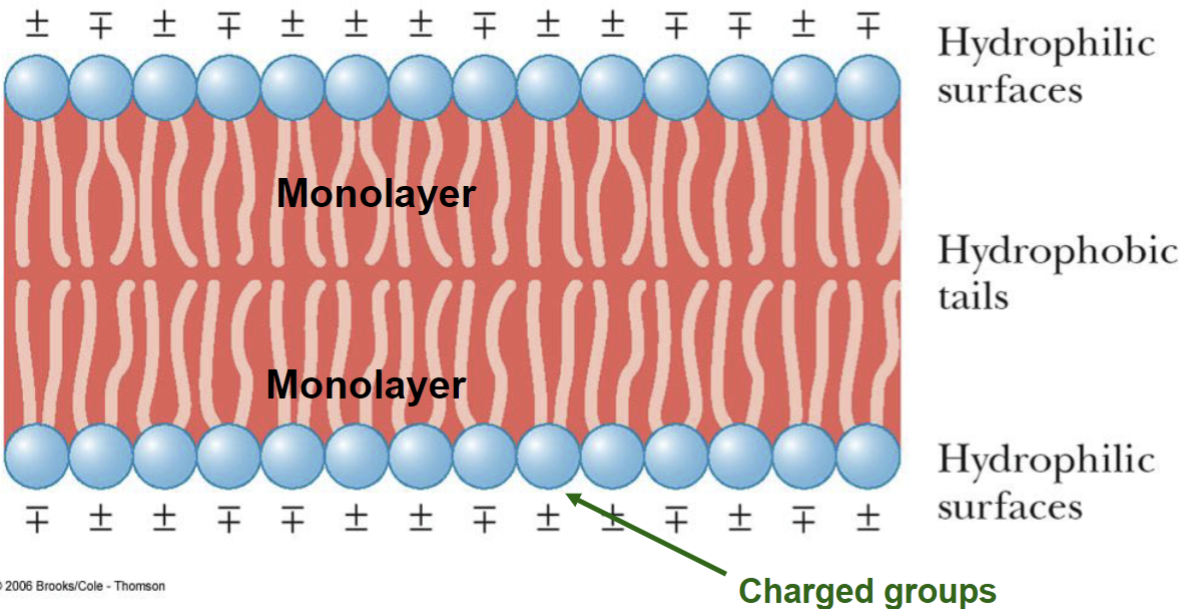
Biological Membranes* (cont.)
• the presence of cholesterol increases rigidity
• with heat, membranes become more disordered; the
transition temperature is higher for more rigid membranes;
it is lower for less rigid membranes
• plant membranes have a higher percentage of unsaturated
fatty acids than animal membranes
• the presence of cholesterol is characteristic of animal
rather than plant membranes
• animal membranes are less fluid (more rigid) than plant
membranes
• the membranes of prokaryotes, which contain no
appreciable amounts of steroids, are the most fluid
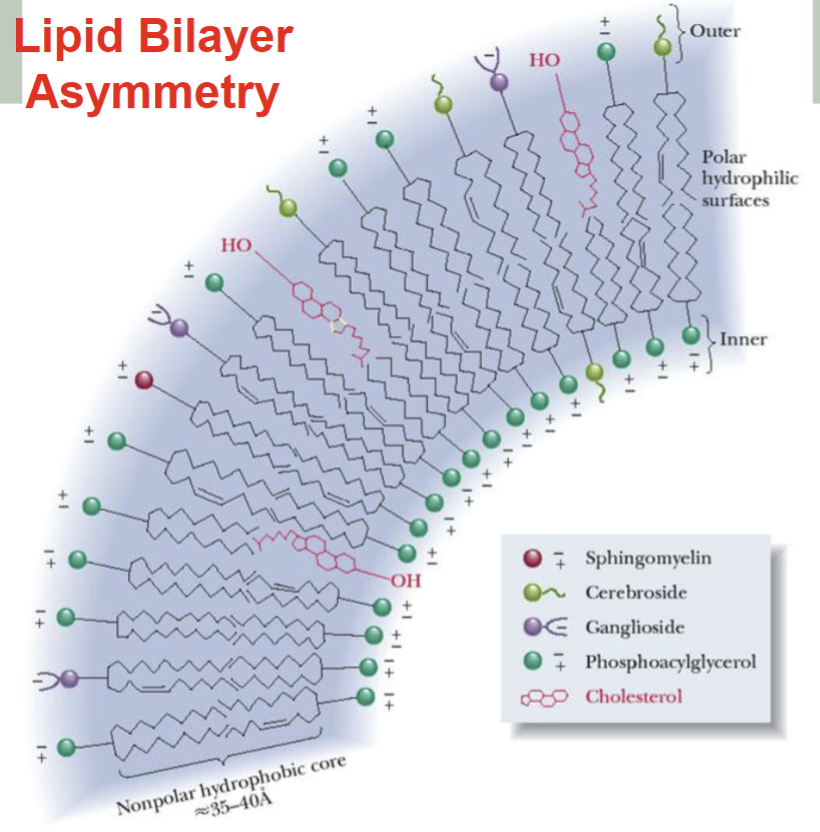
Lipid Bilayer Asymmetry
-The compositions of the outer and inner layers differ.
-The concentration of bulky molecules are in the higher outer layer part that has more space
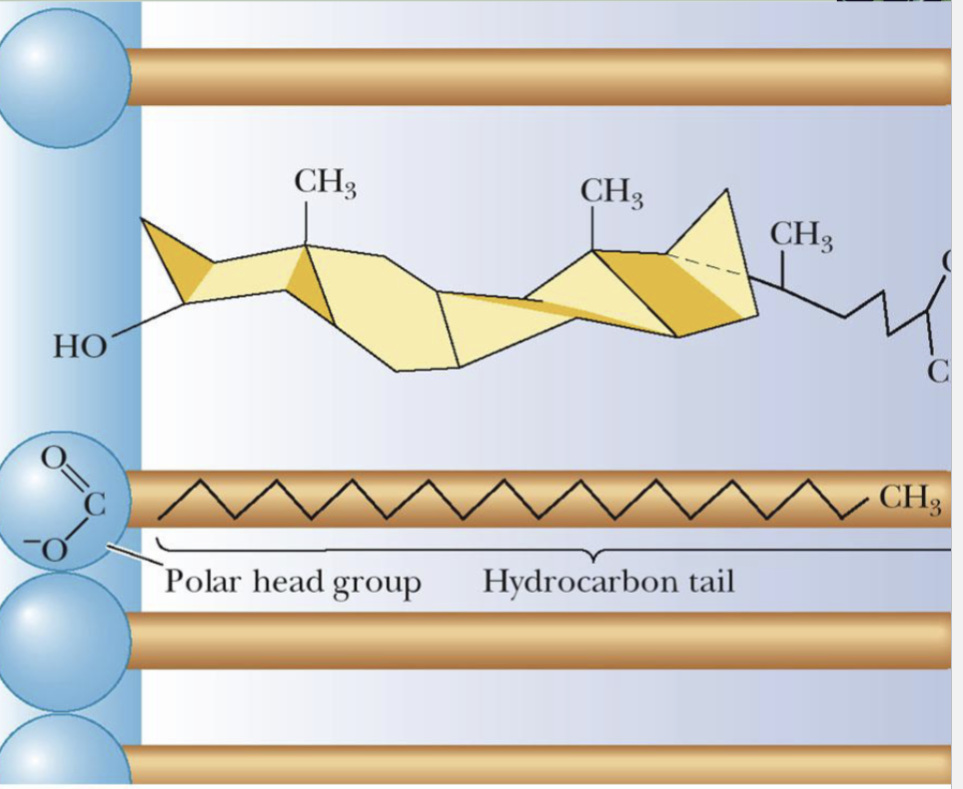
Stiffening of Lipid Bilayer by Cholesterol
Presence of cholesterol in the membrane will reduce the fluidity by stabilizing extended chain conformations in the hydro carbon tails of the fatty acids as a result of Van der Waals interactions
Membrane Proteins
• Functions: transport substances across membranes; act
as receptor sites, and sites of enzyme catalysis
• Peripheral proteins
• bound by electrostatic interactions
• can be removed by raising the ionic strength
• Integral proteins
• bound tightly to the interior of the membrane
• can be removed by treatment with detergents or
ultra sonification
• removal generally denatures them
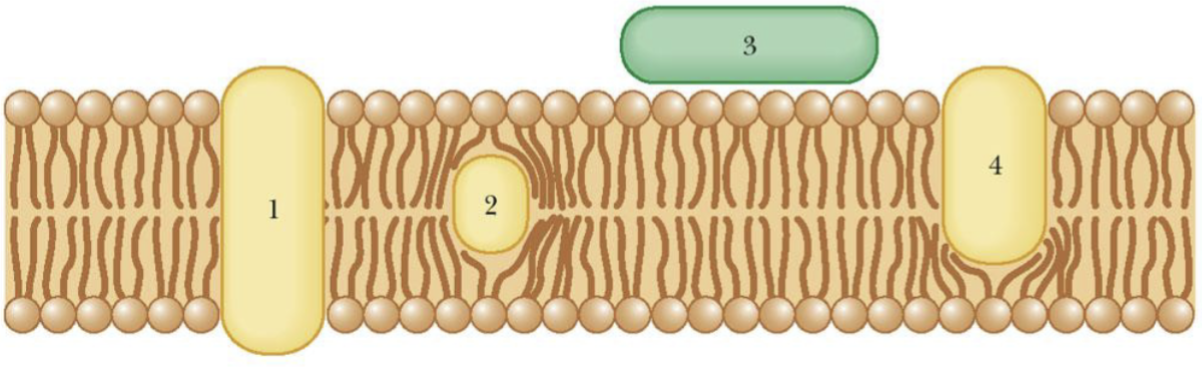
Types of Associations of proteins with membranes
Protein 1 – transverses the membrane
Protein 2 – lies entirely within the membrane
Protein 3 – Peripheral
Protein 4 - projects into the membrane
Integral proteins: 1, 2, 4
Peripheral Protein: 3
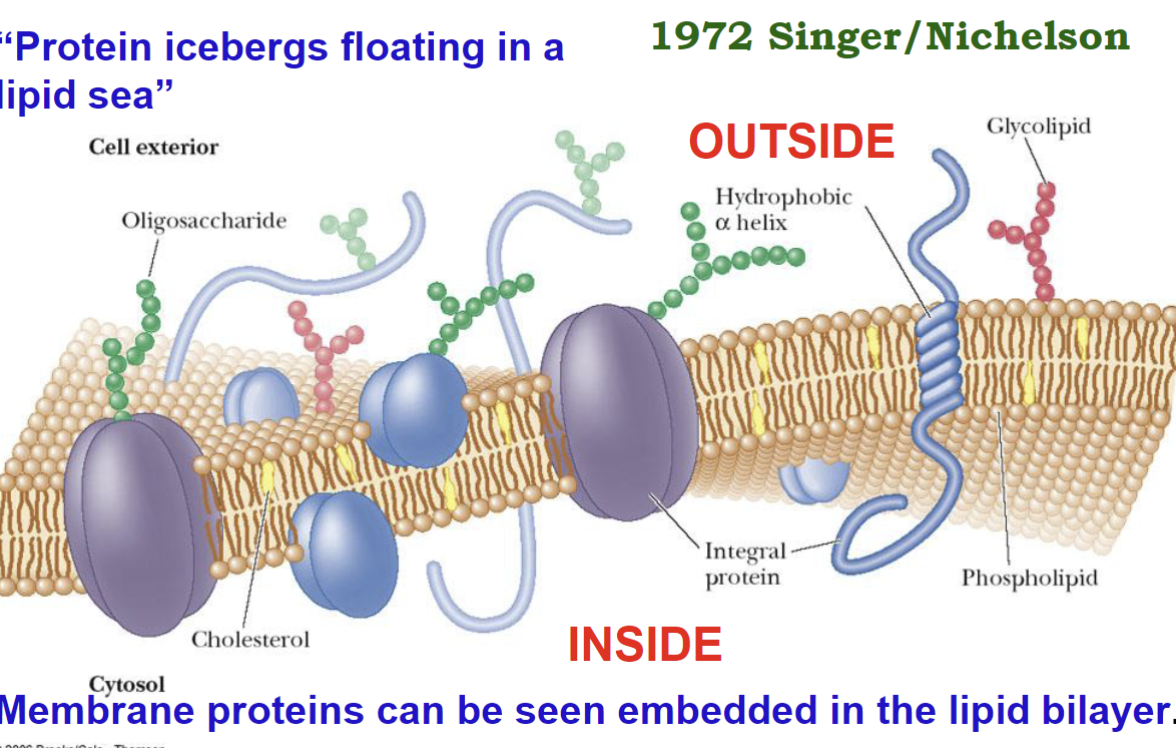
Fluid Mosaic Model
Fluid: lateral motion of components in the membrane; Ex. proteins “float” in the membrane and can move along the plane
Mosaic: components in the membrane exist side-by-side as separate entities
the lipid bilayer with proteins glycolipids, & steroids (e.g. cholesterol) embedded in it
no complexes formed
Passive Transport (membrane transport)
-Driven by concentration gradient
-Simple diffusion: molecules or ions that move through an opening created by channel proteins
-Facilitated diffusion: molecules or ions “carried” across a membrane by carrier protein
Active Transport (Membrane Transport)
A substance moved against a concentration gradient
-Primary Active Transport: transport is linked to hydrolysis of ATP or a high-energy molecule (e.g. Na+/K+ ion pump)
-Secondary Active Transport: driven by H+ gradient
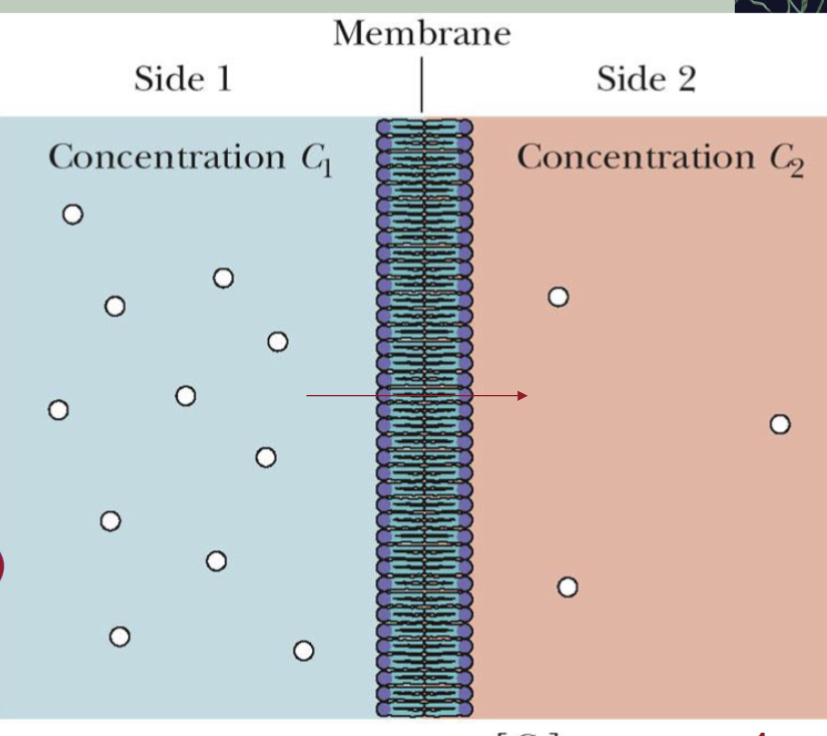
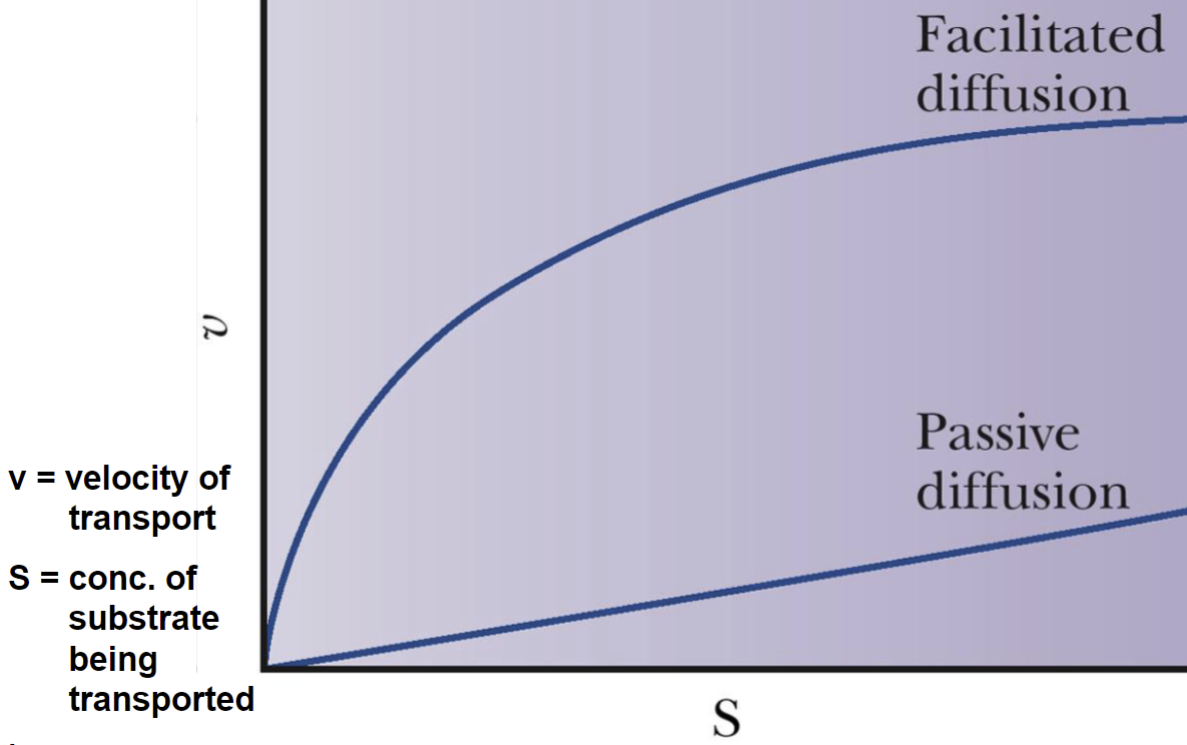
Passive diffusion
an uncharged species across a membrane depends only on the
concentrations (C1 and C2) on the two sides of the membrane.
Small, uncharged molecules:
(e.g. O2, N2, H2O, CO2) can pass through membranes by simple diffusion
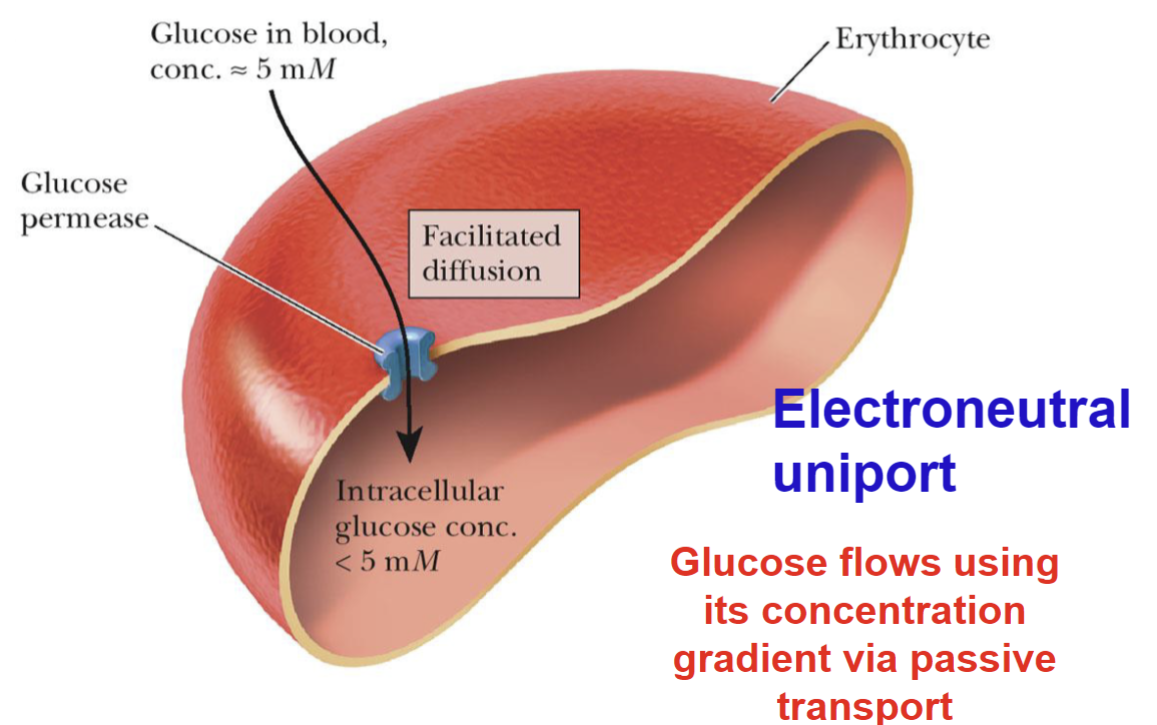
Facilitated Diffusion
Glucose passes into a erythrocyte via glucose permease by facilitated diffusion
Passive vs. Facilitated Diffusion Curve Graph
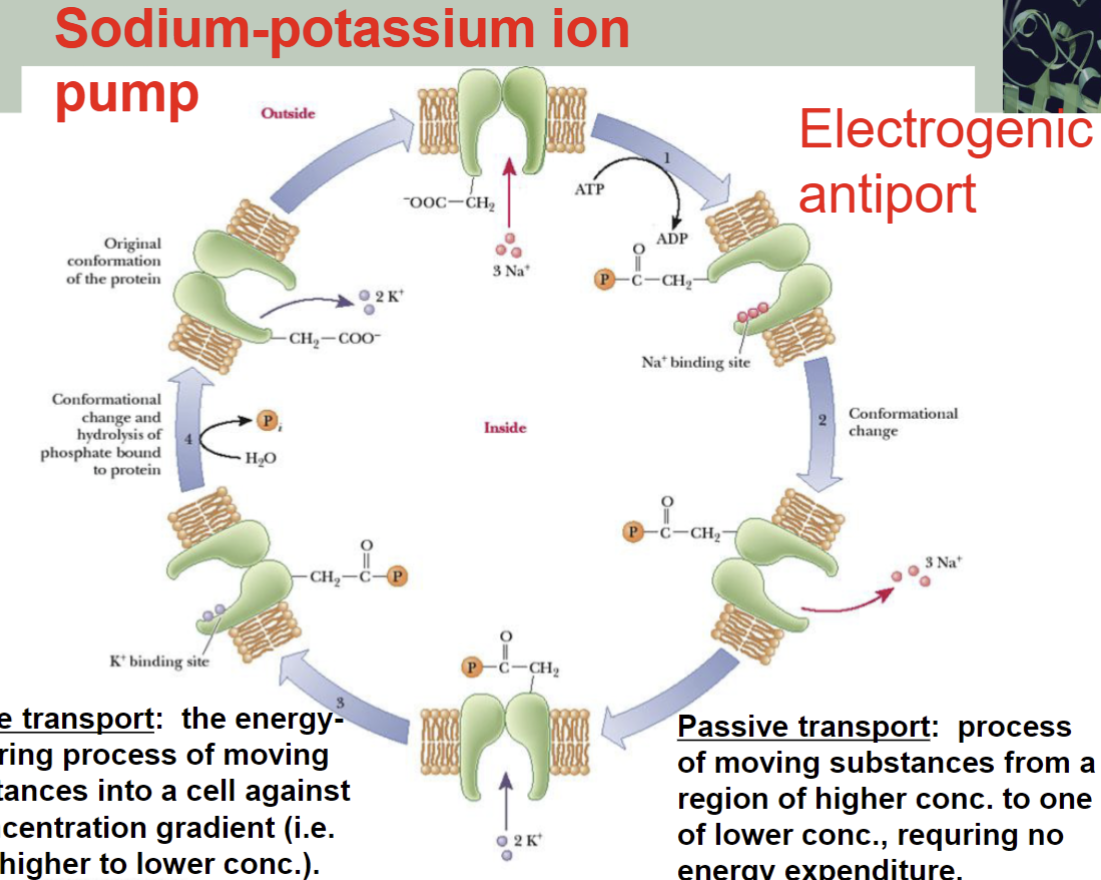
Sodium-potassium ion pump
Passive transport: process of moving substances from a region of higher conc. to one of lower conc., requiring no energy expenditure
Active transport: the energy-requiring process of moving substances into a cell against a concentration gradient (i.e. from higher to lower conc.)
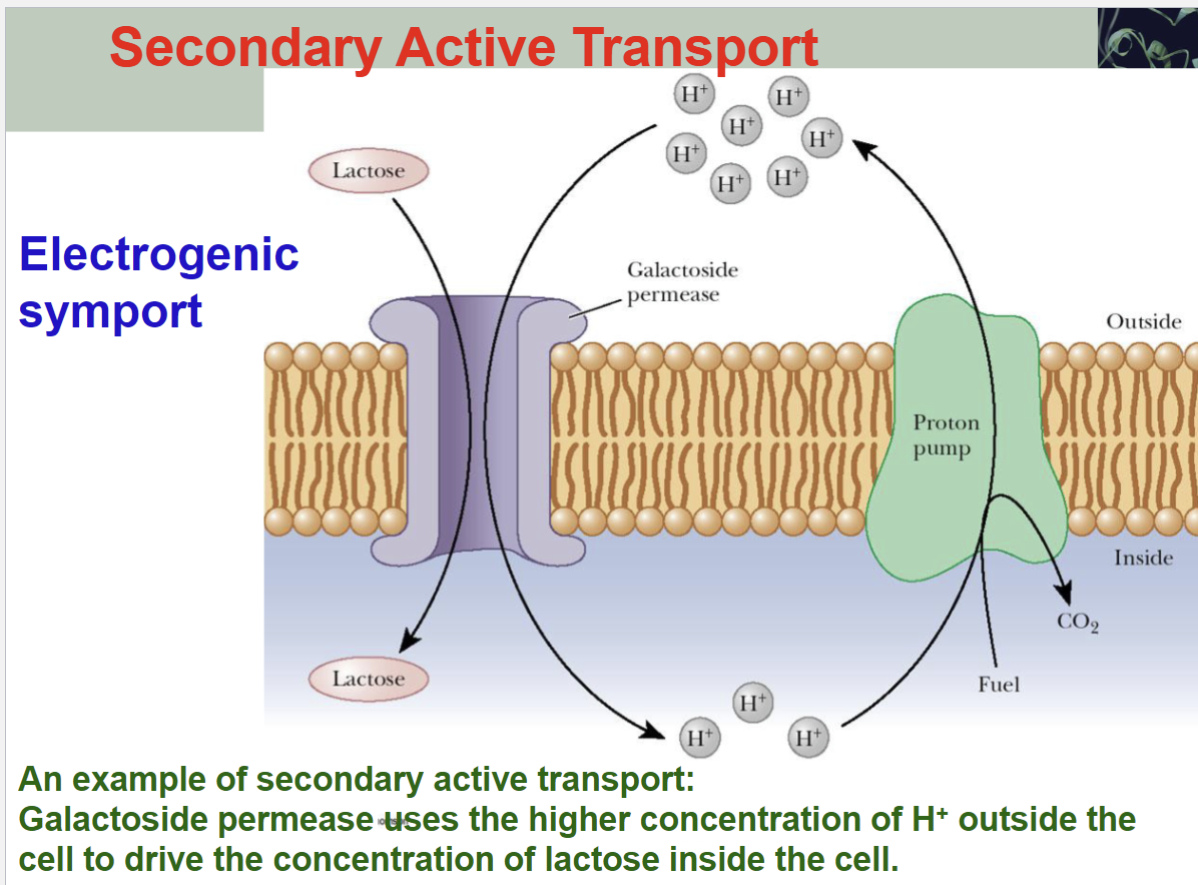
Secondary Active Transport
An example of secondary active transport:
Galactoside permease uses the higher concentration of H+ outside the cell to drive the concentration of lactose inside the cell