1.4 - Covalent Bonding
1/8
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
9 Terms
What substances does covalent bonding occur between?
non metals
What does covalent bonding involve?
a shared pair of electrons between atoms
Why do covalent bonds occur?
to achieve a full outer shell
How strong are covalent bonds?
very strong
How do you draw a dot and cross diagram?
When drawing Dot & Cross diagrams, we only draw the outer shell of electrons:
1. Fill up the outer shell of electrons by spacing the first four as far apart as possible, then pair the last four.
2. The electrons that are unpaired are the ones involved in the covalent bonding.
3. Re-arrange the electrons so the unpaired electrons are facing each other
4. Now join the two atoms together so that the unpaired electrons are being shared
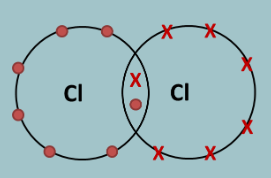
What is a covalent compound?
compounds formed from non metals consisting of molecules
How are two or more atoms held together?
strong covalent bonds
How are molecules held together?
weak intermolecular forces