Chemistry I - Exam 4
1/24
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
25 Terms
Crystalline Solids
have ordered structures
Which species has London dispersion forces as the ONLY intermolecular force?
Ar
When KBr dissolves in water, aqueous K+ and Br- ions results. The force of attraction that exists between K+ and H2O is called a(n) _______ interaction.
Ion-dipole
The intermolecular force(s) responsible for the fact that CH4 has the lowest boiling point in the set: CH4,CH3CH3,CH3CH2CH3,CH3CH2CH2CH3 is/are
London Dispersion forces
Hydrogen bonding is a special case of
dipole-dipole attractions
The predominant intermolecular force in water is
hydrogen bonding
Which of he following molecules has london forces as its only intermolecular force
CH3CH3
what types of intermolecular forces exist between CH3OH and H20
dispersion forces, dispole-dispole, and hydrogen bonding
what types of intermolecular forces exist between C2 and CCI4
dispersion forces
the phrase “like dissolves like” refers to the fact that
polar solvents dissolve polar solutes and nonpolar solvents dissolve nonpolar solutes
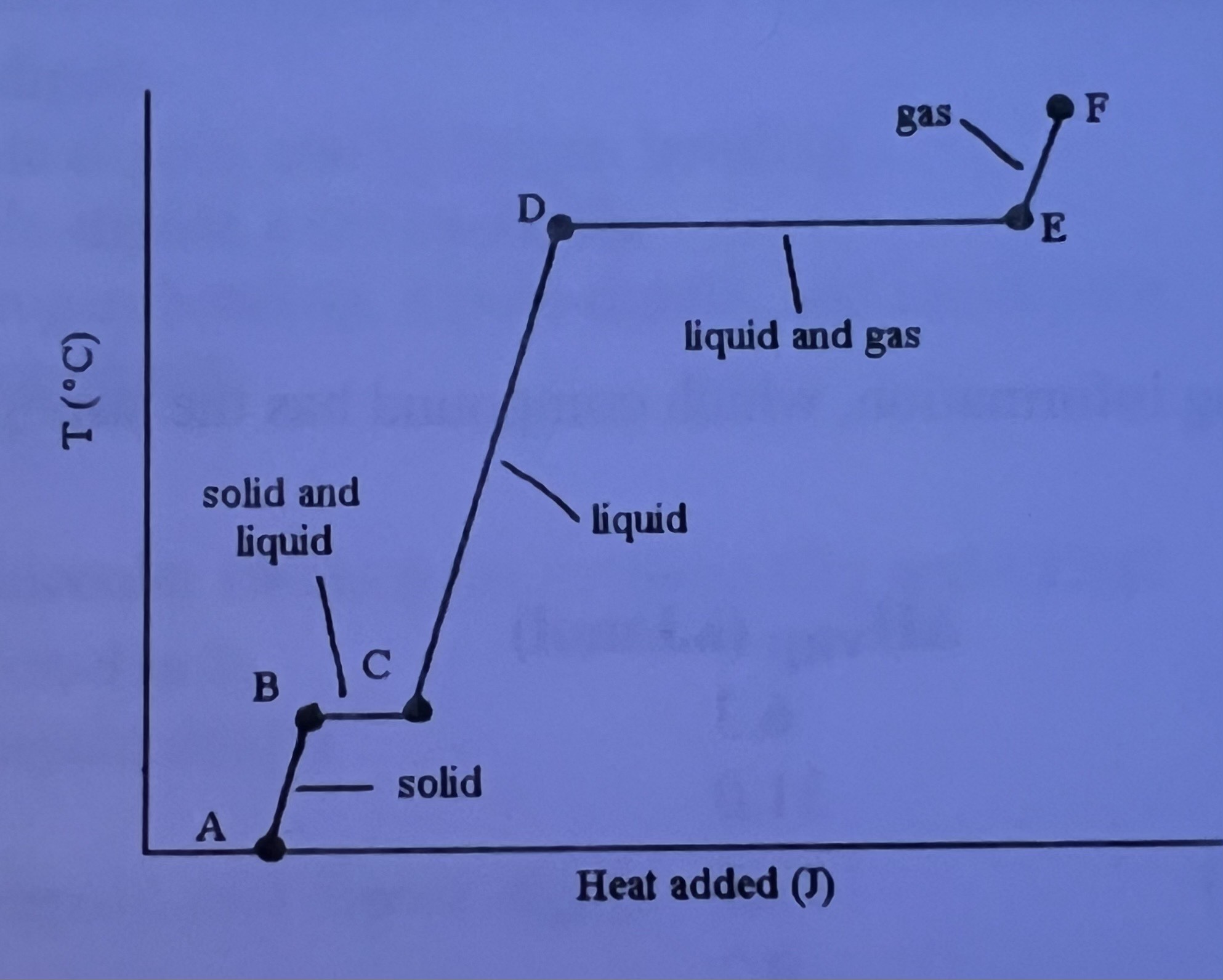
The _______ is are associated with the heat energy being used up to increase distance between molecules
Phase change B → C and D → E

Based on the following information, which compound has the strongest intermolecular forces?
Ethanol
The predominant intramolecular forces in KBr is
ionic bonding
diamond and graphite, two substances that differ in the arrangement of their atom but only contain the element carbon, can be referred to as
Allotropes
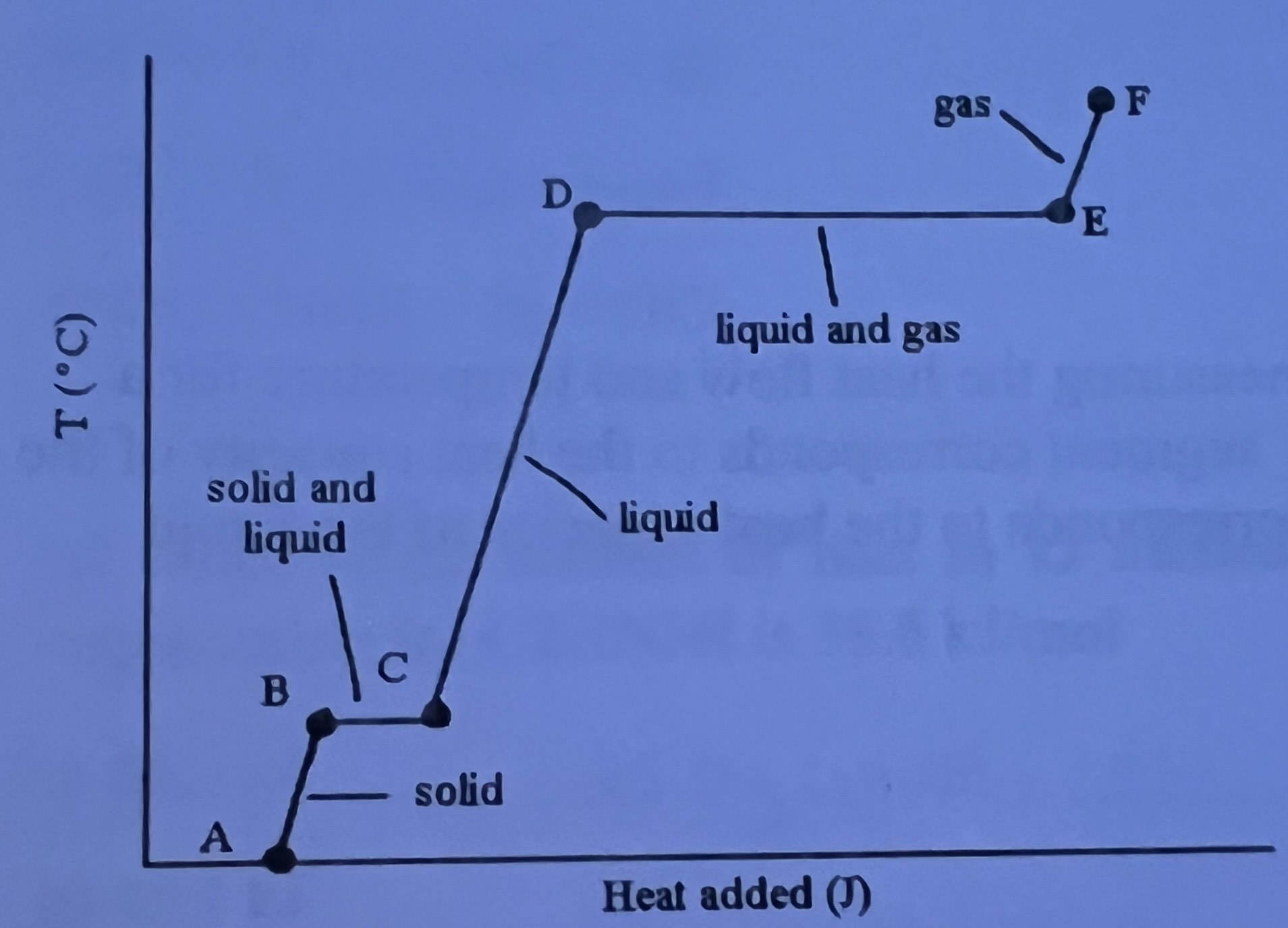
The heating curve shown was generated by measuring the heat flow and temperature for a solid as it was heated. The slope of the _____ segment corresponds to the heat capacity of the solid and slope of the ____ segment corresponds to the heat capacity of the liquid respectively.
AB and CD
What is the molar concentration of potassium bromide in a solution prepared by dissolving 4.57 g of potassium bromide in 597 g aqueous solution? Kbr MM= 119.02 g/mol, dwater = 1.0g/mL
0.0643 M
what is the correct sequence of the substance NaCI, H2, CO, and HCI in order of increasing boiling point
H2 < CO < HCI< NaCI
what is the amount of heat in KJ needed to evaporate 65.0 g of C2H5OH? The heat of vaporization for C2H5OH is 38.6 kJ/mol
54.5 kJ
a substance that solidifies into a random molecular arrangement is referred to as an amorphous solid
True
Molarity is defined as the
moles solute/ liters solution
what is the molar concentration of a solution which is prepared by dissolving 12.0 grams of NaCI in 240. mL of aqueous solution
0.862 M
a solution contains 11% by mass of sodium chloride. This means that
100 g of the solution contains 11 g of sodium chloride
which molecules has hydrogen bonding as the predominant intermolecular force
CH3CH2OH
when a liquid reaches a temperature at which its vapor pressure is equal to one atmosphere, the liquid has reached its ___
normal boiling point
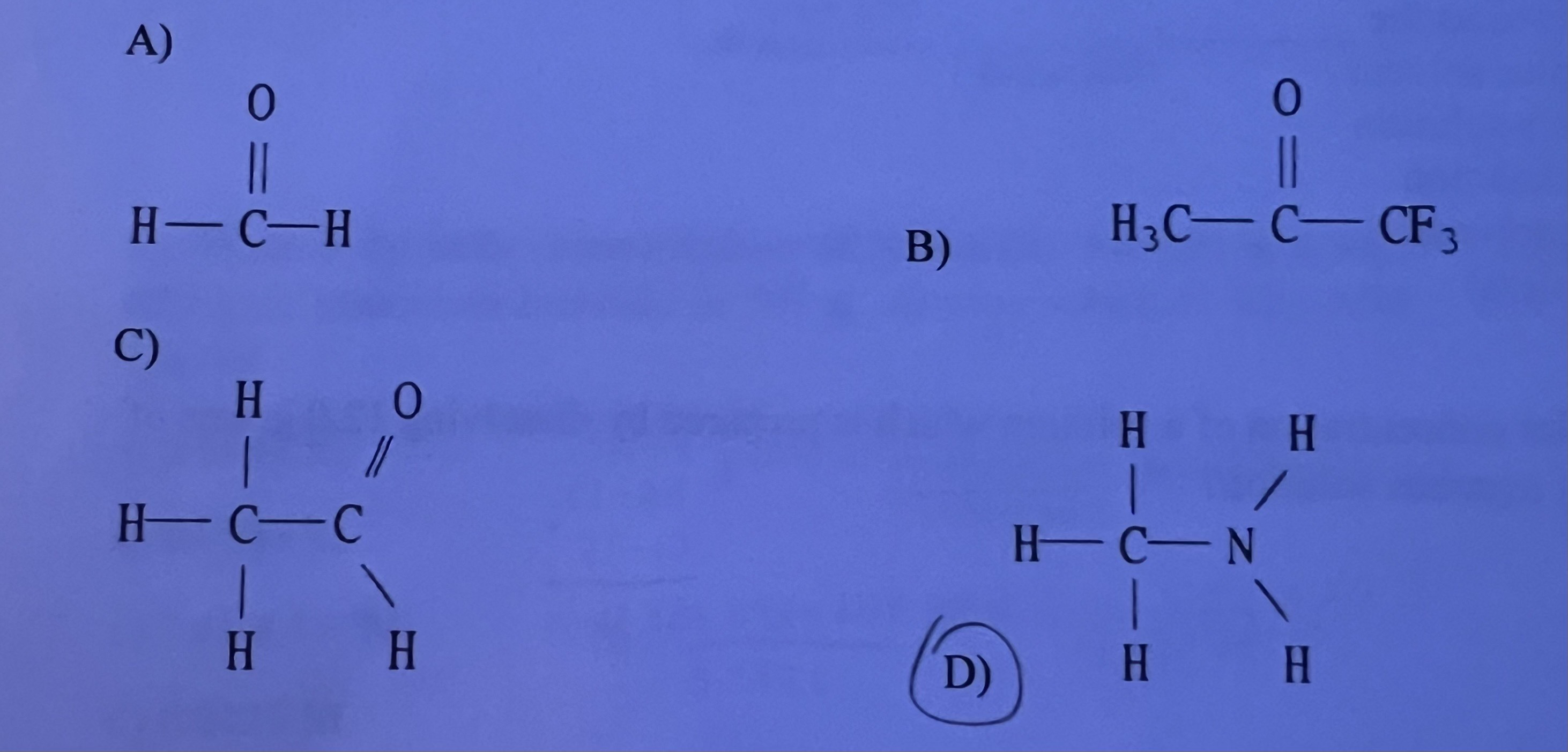
Which of the following substances will have hydrogen bonding as one of its intermolecular forces?
D