CHEM 1151: Topic 2, Quantum Mechanics
1/98
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
99 Terms
What is a wave?
A wave is a propagating dynamic disturbance of one or more quantities
What is a periodic wave?
A wave that oscillates about an equilibrium value
What is a traveling wave?
A wave that moves through space and time in one direction (e.g., sound, light, ocean waves)
What is a stationary wave?
A wave that oscillates in place (e.g., a guitar string)
What is equilibrium? How is it identified, located, and measured?
Equilibrium is the baseline value about which a wave oscillates; it is identified as the zero-displacement (mean) line in a waveform
What is an equilibrium value (periodic wave)?
The equilibrium value is the central value that the oscillation occurs about in a periodic wave
What are some examples of periodic traveling?
Sound waves, light waves, ocean waves
What are some examples of stationary waves?
Vibrations of a guitar string
What is a wavelength λ?
The distance between two equivalent points in a wave, such as crest-to-crest.
How are wavelengths measured & what are its units?
It is measured in units of length, typically nanometers (nm) or meters (m)
What is frequency 𝜈?
The number of oscillations that pass through a point per unit time.
How is it measured & what are its units?
It is typically measured in Hertz (Hz), which is equivalent to s-1. It is typically measured in Hertz (Hz), which is equivalent to min-1, hr-1
How are wavelength and frequency linked? What is the relationship (inverse or direct); are they independent or dependent on each other?
Wavelength and frequency are inversely related by the equation λν=c, where c=2.998×108 m/s (speed of light). They are not independent; as one increases, the other decreases
What is Newtonian Physics?
The accepted framework, emphasizing determinism and continuity for classical quantities
What is determinism?
The concept that a system’s future history can be predicted if all initial conditions and acting forces are known
What are continuous quantities? What are some examples?
This takes on any value within a range, such as energy, momentum, and acceleration
What category does light fall under?
Light (radiation) was regarded as a wave
What is Maxwell’s Equation & what behavior did it explain?
A set of four fundamental laws of classical electromagnetism that describe how electric and magnetic fields are generated and altered by each other and by charges and currents. It explained electric and magnetic phenomena
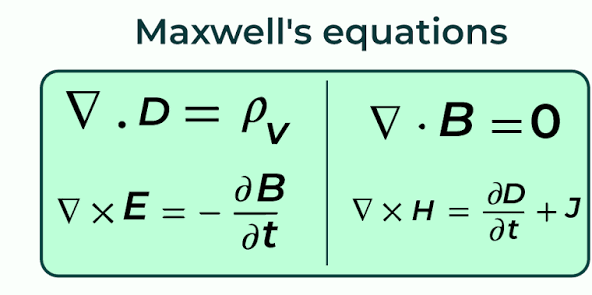
Why were the ideas of Newtonian physics, radiation (waves), and Maxwell’s eq relevant to macroscopic objects?
They described and predicted the behavior of macroscopic objects very well
Why according to classical physics is Rutherford’s Model not possible?
Accelerated electrons in Rutherford’s atom would emit energy and spiral into the nucleus, a collapse not observed in reality
Describe in general terms what the “ultraviolet catastrophe” is.
Classical physics, via the Rayleigh-Jeans Law, predicted that radiated energy from a heated object would become infinite at short wavelengths (ultraviolet), contrary to experimental observation
Equation: Intensity=8πkT/λ4
What are oscillators?
Atoms vibrating around equilibrium positions due to thermal energy; in Planck's theory, these are the emitters of quantized energy
What equation represents the fundamental value of energy at each frequency?
E=hν or *E=h⋅c/λ
*Derived & manipulated from λv=c
What is the fundamental value of energy called?
A quantum of energy
What is Planck’s constant? What is its value?
A fundamental universal constant in quantum mechanics that defines the smallest possible "packet" of energy, called a quantum, and relates a photon's energy to its frequency through the equation E = hν. h=6.626×10−34J⋅s ; Value: h=6.626×10-34J⋅s
What conditional relationship is represented by oscillators and the fundamental value? What is the conditional relationship called?
Only integer multiples of the quantum can be emitted/absorbed. The total energy of the system is decomposed into an integer number of these oscillators. The relationship is called quantization
How and why is Max Planck’s hypothesis significant?
Planck’s quantization matched observed blackbody radiation, solving the ultraviolet catastrophe
What is quantization?
Energy is emitted/absorbed in discrete units E=n⋅hν, not continuously
Why is quantization not possible according to classical physics?
Classical physics regarded energy as continuous, allowing any arbitrary value and no energy "quanta"
What is the photoelectric effect?
Emission of electrons from a metal when light of sufficient frequency shines upon it
Why couldn’t classical physics explain the photoelectric effect?
It couldn’t predicted energy absorption should depend on light intensity, not frequency, but experiments showed otherwise
What is the maximum velocity of electrons dependent on?
Dependent on the color (frequency) of the incident light
Can all colors of light (frequency, wavelength) produce the ejection of electrons? If not, why?
No, only frequencies at OR above the threshold frequency (color-dependent) can eject electrons
What linear-dependent relationship is present in the photoelectric effect? What are some examples/results of this relationship?
Kinetic energy of ejected electrons depends linearly on light frequency (KEmax =hν−Φ), not on intensity
Does light intensity play a role in the photoelectric effect? Explain.
Yes, it's proportional to the number of emitted electrons, not their energy; if its below threshold frequency, no electrons are ejected, regardless of intensity
How does the threshold wavelength (𝜆₀) correspond to a threshold frequency (𝜈₀)? What happens with frequencies above or below it?
For a material, the threshold wavelength (𝜆₀) or equivalently threshold frequency (𝜈₀) is the cutoff:
Φ=h𝜈0Φ=h𝜈0, only frequencies above this threshold can eject electrons
Below 𝜈₀: no electrons; above 𝜈₀: electrons are ejected and have kinetic energy increasing linearly with frequency
What are photons?
Photons are massless particles of light, each with quantized energy (E=hν)
How do photons relate to the wave-particle duality? What is their energy equation?
Photons support the wave-particle duality by exhibiting both wave-like properties, such as interference and diffraction, and particle-like properties, like collisions and discrete energy packets. Their energy is described by the equation E = hf or E = hc/λ, where E is energy, h is Planck's constant, f is frequency, c is the speed of light, and λ is wavelength
How much can an electron in a material hold/absorb?
An electron can absorb only one photon at a time
In what two ways does light act/function? Why/how?
Light acts as both a wave (interference/diffraction) and as particles (photon-based processes like the photoelectric effect)
How are electrons in a metal held? What is it called, and why are they held with a certain amount of energy?
Electrons are held by a certain energy called the work function (Φ), which is the minimum energy needed to eject an electron from the metal
How is work function represented? What are its units?
Work function is denoted Φ, measured in Joules (J) or per mole (J mol⁻¹)
What process is required for an electron to be released from a metal?
A photon of energy greater than Φ must be absorbed to release an electron
What is the difference between work function and threshold frequency?
The work function (Φ) is the minimum energy needed to eject an electron from a metal (think: the energy barrier for escaping the material)
The threshold frequency (ν₀) is the minimum frequency of light required to provide a photon with at least the work function energy (Φ=hν0)
To get electrons out, it’s necessary to…?
Provide a photon with energy at least equal to the work function Φ
What happens if energy E = work function (Φ)?
Electrons are ejected but with no kinetic energy since all photon energy is used to free the electron (KE=0)
What happens if energy E > Φ?
The excess energy appears as the kinetic energy of the ejected electron: KE=E−Φ
Conservation of energy: What happens if E < Φ?
No electrons will be emitted because the photon does not have enough energy to overcome the energy barrier
Why can’t kinetic energy be negative? If kinetic energy is negative, what happens?
Kinetic energy physically cannot be negative; a negative result means no electron is ejected → there simply isn’t enough energy
How do the natural threshold and a limiting frequency relate?
The work function sets a natural threshold that establishes the minimum (threshold) frequency needed for emission: Φ=hν0
Kinetic energy of the ejected electrons is a linear function of the light frequency.
KEmax=hν−Φ: As frequency increases above ν₀, KEmax increases linearly
What is proportional to the number of photons? Describe the relationship.
The intensity of light; higher intensity means more photons, which means more electrons can be ejected (if frequency is above threshold)
What does the energy of a photon depend on?
A photon’s energy depends only on its frequency: E=hν
Does the production of photoelectrons depend on the intensity? Why or why not?
Only if the frequency is above threshold; increasing intensity increases the number, but not the energy, of ejected electrons. Below threshold, increasing intensity does nothing
What impact does the intensity of the light have on the photoelectric effect?
Above threshold, it increases the number of ejected electrons; below, it has no effect
How is wave-particle duality expressed? Is it limited to how it’s exhibited? Explain.
All matter exhibits wave-particle duality; it is not limited to light—every object has an associated wavelength (λ=h/(mv)), but this is observable only for very small particles, not macroscopic objects
What concept did de Broglie hypothesize/assert?
He suggested every object has a wavelength associated with it, depending on mass and velocity
What formula is associated with de Broglie’s concept?
λ=h/(mv)
What does de Broglie’s formula do that’s different from classical physics?
It unites particle and wave concepts, showing “classical” particles (like electrons) also have a wave nature
Why do large, everyday objects always appear to behave as particles, while small particles like electrons can exhibit observable wave-like properties?
For macroscopic objects (large mass), the wavelength is so tiny it’s unobservable, so they appear to behave as particles, while small particles (e.g. electrons) have observable wavelengths and quantum behavior
What is a discrete emission?
Only photons of certain specific wavelengths (or energies) are emitted or absorbed by atoms; not all wavelengths are allowed
What formula did Johann Balmer use to illustrate how frequencies of lines in the absorption spectrum of H₂?
ν=8.224×1014Hz(1/4−1/n2), with n=3,4,5,…
What did Johannes Rydberg show through Johann Balmer’s formula?
Rydberg generalized Balmer’s formula to describe all spectral lines of hydrogen
What is Johannes Rydberg’s formula?
When R=1.097×107m-1
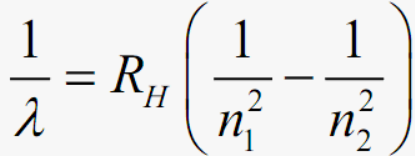
What does empirical mean?
“Empirical” means the formula fits observed data but wasn’t derived from theory
What does n2>n1 mean?
It specifies that for absorption, transitions must go from a lower initial (n1) to a higher final (n2) energy state
What did Niels Bohr’s model of quantization entail?
Electrons move in allowed orbits with quantized energies, and do not emit energy while in these orbits
What could the electrons do or not do?
Electrons can “jump” between allowed energy levels but cannot exist in between them
What is this condition called?
This restriction is an “ad hoc postulate”—a rule created to fit observations
What is an energy level?
A quantized energy state for an electron in an atom
What is the expression of the energy level?
En=−2.178×10-18J⋅(1/n2)
What does n represent? What are its restrictions/limitations?
n is the principal quantum number (n=1,2,3,...). Only positive integers allowed
What happens if an electron is in an orbit?
It does not emit energy
What is an ad hoc postulate?
A rule added to save the experiment from being disproven, even if lacking a deeper theoretical justification
Can electrons move/“jump” between energy levels? If so, how?
Yes, by emitting or absorbing a photon with energy equal to the difference between levels
Can they exist between them? Why or why not?
No, only in specific quantized levels
When will an energy level be at its highest? What does this correspond to?
When n→∞, the energy approaches zero (unbound electron)
What is the value of E∞?
E∞=0, which means the electron is removed from the atom
What is the value of n that corresponds to the lowest possible value of the energy in Bohr’s model?
The lowest energy level is for n=1, called the ground state
What conditions do electrons have concerning energy levels?
Electrons reside only in allowed, quantized energy levels
What happens when electrons are in transition?
They absorb or emit a photon, changing levels
What equation represents the absorption or emission of photons of energy?
For a photon: E=hν=hc/λ
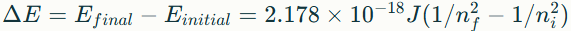
Energy (in J) is absorbed or released for the transition of what?
For an electron moving between energy levels
To have an absorption, what must be met?
Electron moves from a lower to a higher level (nf>ni)
To have an emission, what must be met?
Electron moves from a higher to a lower level (ni>nf)
Why must the difference in energy levels equal the energy of the absorbed photon?
Conservation of energy: the energy supplied by the photon must exactly match the energy required for the electron to change levels
If a photon is absorbed during an electronic transition, what must its energy be equal to?
The difference in energy between the two involved energy levels
What does Rydberg’s formula specify?
It predicts the wavelength or frequency of light for transitions between any two energy levels in hydrogen
According to Rydberg’s formula, what condition must be satisfied for absorption to occur?
n2>n1 (final is higher than initial)
If an electron moves from n=5 to n=2, will the photon wavelength be different than from n=2 to n=5?
Yes; n=5→2 is emission, n=2→5 is absorption; the photon’s wavelength is the same, but the physical process (absorption vs emission) differs in direction
Can you use Rydberg’s formula for emissions? If so, what does the negative sign indicate?
Yes, a negative value indicates emission (downward transition), not a negative wavelength
What is the Rydberg formula for absorption?

What is the Rydberg formula for emission?
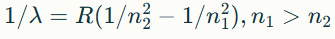
Pro tip: always identify the type of problem (absorption vs emission) and use the correct formula.
Confirm the electron’s direction and match formulas accordingly
What does the presence of the 1/n2 factor in the energy expression imply about the spacing of electron energy levels?
Energy levels are not equally spaced; they get closer together as n increases
What happens to the separation between adjacent energy levels as n increases?
The separation between adjacent energy levels decreases; higher levels are more closely spaced
What does this decreasing separation between energy levels mean for transitions?
Transitions between high-n levels involve less energy than transitions between low-n levels
Which requires more energy: promoting an electron from n=2 to n=3, or from n=5 to n=6? Why?
n=2→3 requires more energy because lower levels are much farther apart
Why does a photon emitted in a transition from n=3 to n=1 have more energy than one emitted from n=4 to n=2, even though both involve a two-level change?
Because the spacing is larger between lower energy levels; the bigger “jump” in energy at lower n means the photon is more energetic