Strong Acids and Bases
1/43
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
44 Terms
HCl
strong acid
HBr
strong acid
HI
strong acid
HClO4
strong acid
HNO3
strong acid
H2SO4
strong acid
LiOH
strong base
NaOH
strong base
KOH
strong base
RbOH
strong base
CsOH
strong base
Ca(OH)2
strong base
Sr(OH)2
strong base
Ba(OH)2
strong base
Strong electrolytes are
strong acids, strong bases, soluble ionic salts
Do strong electrolytes conduct electricity?
Yes, a lot
Do strong acids dissociate completely?
Yes
Do strong bases dissociate completely?
Yes
Do weak acids and bases dissociate completely?
They partially dissociate, not fully
soluble (aq)
dissolves in water
HF is it weak or strong?
Weak
Arrhenius acid
Produces H+ ions when dissolved in water
Arrhenius base
Produces OH- ions when dissolved in water
Bronsted-Lowry acid
proton donor
Bronsted-Lowry base
proton acceptor
water can be an acid or base
Amphiprotic
NH3
Weak
HCN
Weak Acid
CH3COOH
weak acid
H2CO3
weak acid
Ions are
charged atoms or molecules that have gained or lost electrons
Isotopes are
atoms of the same element with different atomic masses
Cation
A positively charged ion
Anion
A negatively charged ion
Why is Hydrogen H+
Because it gives 1 valence electronto become positively charged
ionic compound
A compound that consists of positive and negative ions
ionic compound
a compound composed of positive and negative ions
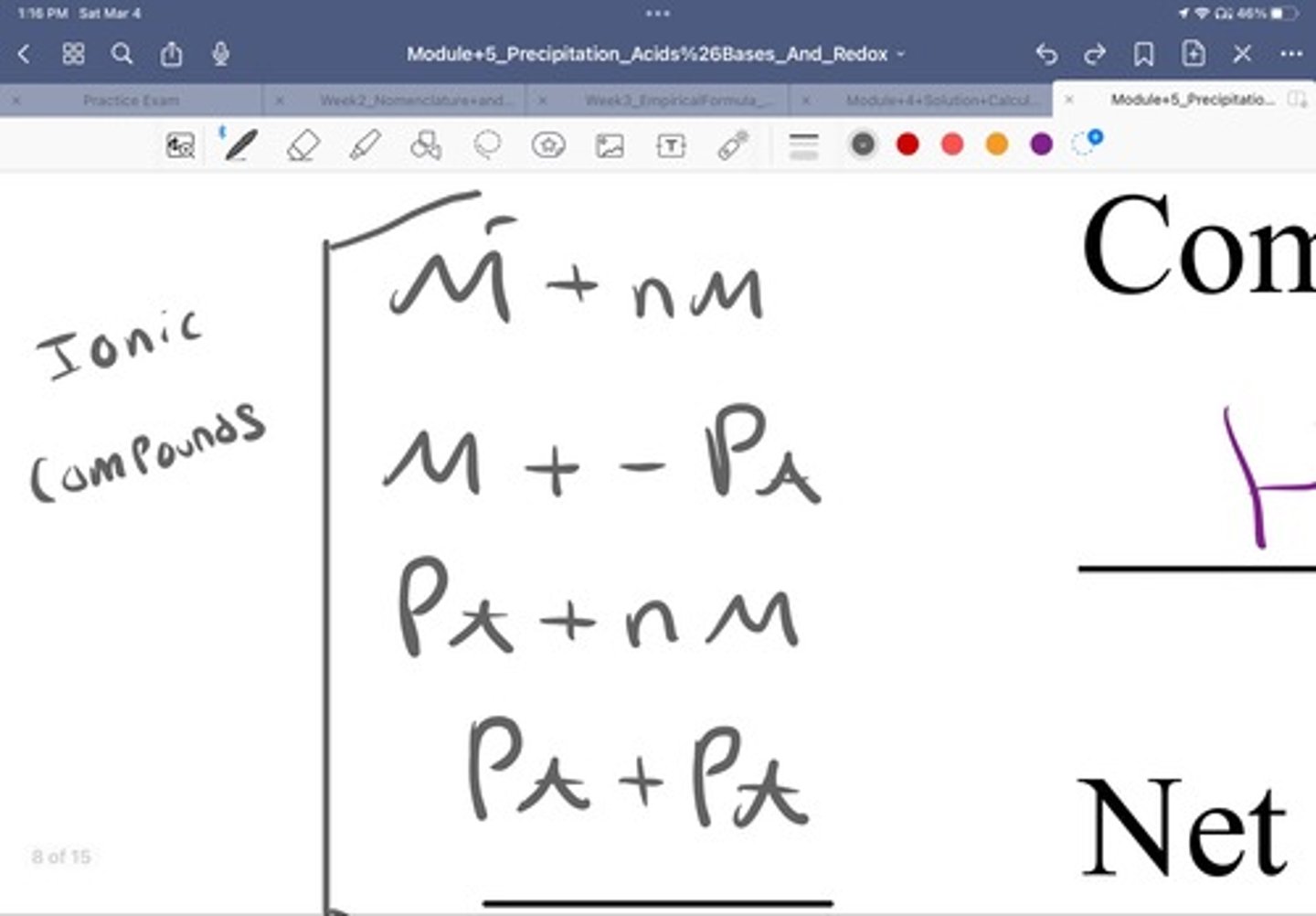
Is an ionic compound a metal and a non metal anion?
Yes
Is an ionic compound a metal and a anion polyatomic ion?
Yes
Is an ionic compound a polyatomic ion and a nonmetal anion?
Yes
Is a cation polyatomic ion and a anion polyatomic ion a ionic compound?
Yes
Rule One Solubility rules in water
All ammonium, and Group one elements are soluble
Rule 2 Solubility rules in water
All nitrate (NO3-) and Nitrite (NO2-) are soluble in water
Rule 3 solubility rules in water
All chloride (Cl-), bromide (Br-), and iodide (I-) salts are soluble except Ag+, Pb+2, and Hg2^2+