C4 Calculations
1/19
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
20 Terms
RFM =
sum of all RAMs in compound
RFM and RAM also written as
RFM = Mr
RAM = Ar
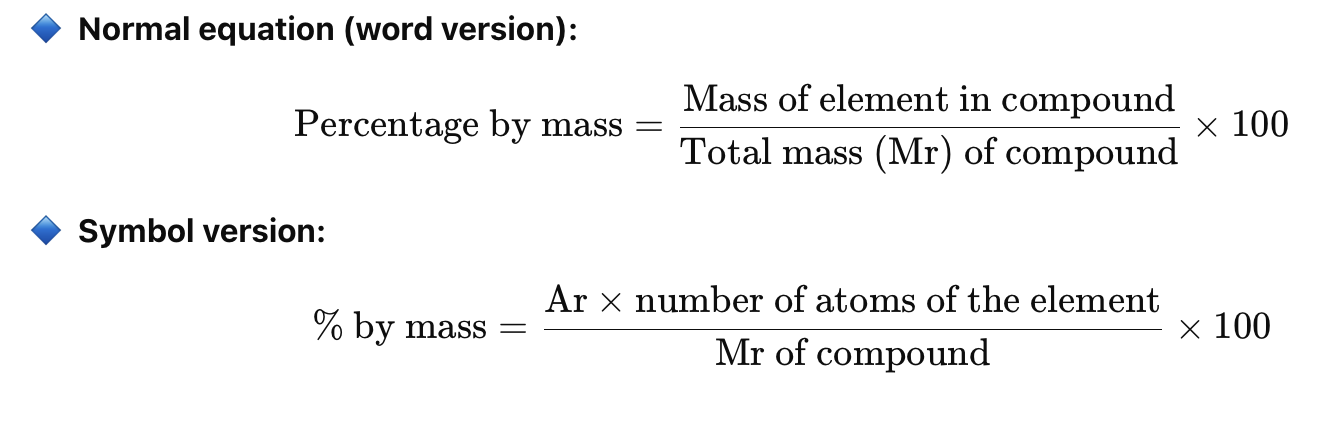
% Mass of an element in a compound =
avagadros constant =
6.02×1023
what does avogradros constant mean
1 mole of a substance = 6.02×1023 particles of the substance
particles could be atoms, molecules, ions or electrons
moles equation
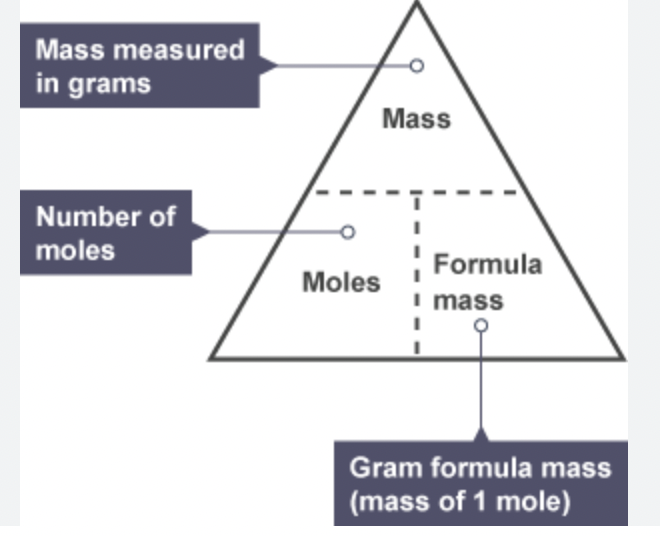
mass is always __
if mass seems to change…
conserved
has has been involvedc
we can’t account for oxygen in air etc
what does burning in air mean
reacting with oxygen
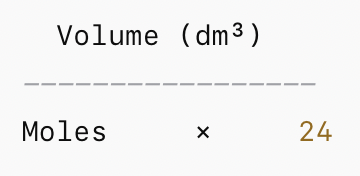
volume of gas equation
volume = moles x 24
TRIANGLE →
Concentration using mass
conc = mass/volume
conc using moles
conc=moles/volume
unit for conc
g/dm3
1dm3 = ___ cm3
1000cm3
dm3 → cm3 = ?
x1000
when doing mol/dm3 to g/dm3 use..
mass = moles x Mr
1dm3 also equals
1 litre
atom economy equation
atom economy = RFM of useful products / RFM of all reactants
convert to %
the higher the atom economy the..
‘greener’ the process
% yield is always under 100% because
reaction could be reversible so some products turn back into reactants
side reactions can occur
you lose product every time you change the container/filter it
% yield equation
% yield = mass of product actually made / maximum theoretical mass of product