Module 4 Flashcards
1/147
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
148 Terms
Inflammation
Movement of fluid and leukocytes from blood into affected tissue in response to injury or infection
May be chronic or acute
Cardinal signs of infection
redness
heat
swelling
pain
Heat/redness
caused by increased blood flow to tissue (called hyperaemia or erythema)
Swelling
increased fluid movement from blood into the tissue (called exudation)
Pain
increased sensitivity of pain receptors (called hyperalgesia)
Oedema
accumulation of fluid extra-vascularly in tissue
Exudate
oedema fluid with high protein content resulting from increased vascular permeability due to inflammation
Pus
inflammatory exudate containing viable and dead neutrophils, cell debris, viable and dead microorganisms, protein, lipids, DNA
Suppuration
the formation of pus
Steps of acute inflammation
Dilation of small vessels = increased blood flow
Increased vessel permeability = plasma protein and leukocytes can leave circulation
Emigration of leukocytes into extravascular tissue and focal area of injury
How exudate is formed in acute inflammation (Injury)
Injury → formation of vasoactive mediators → vasodilation (in arterioles) and endothelial contraction (in post-capillary venules) → hyperaemia and increased hydrostatic pressure (in aterioles) and increased vascular permeability to proteins (in post-capillary venules) → fluids, salts, antibodies, neutrophils move into affected tissue (= EXUDATE)
How exudate is formed in acute inflammation (Infection)
Infection → formation of chemotactic factors → upregulation of endothelium adhesion molecules (in post-capillary venules) and Neutrophil activation → Neutrophil margination and migration → fluids, salts, antibodies, neutrophils move into affected tissue (= EXUDATE)
Vasoactive Mediators
cause vasodilation in arterioles (hyperaemia and increase hydrostatic pressure follow)
causes endothelial contraction in post-capillary venules (increase permeability to protein follows)
Chemotactic factors
Increase adhesion molecules on endothelium of post-capillary venules
Increase neutrophil margination and migration
Exudate components
Fluid: for dilution of toxins and increase flow into lymphatic system to flush out infection
Plasma proteins: antibodies, complement system, and fibrin system components
Neutrophils: for destruction of foreign microorganisms
Changes in vascular flow
Vasodilation
earliest change - caused by histamines on vascular smooth muscle
affects arterioles, then capillary beds
causes heat + redness
Increased permeability
first transdudate forms (protein-low fluid)
second exudate enters tissue (protein-rich fluid)
Vascular congestion
increased viscosity of blood
slow moving blood allows for protein leakage
Process of leukocyte migration
Activation of leukocytes
Rolling of leukocytes along vessel cell membrane (with the help of selectins)
Firm adhesion of leukocytes on vessel cell membrane (with help of integrins)
Entering of leukocyte into tissue via diapedesis (with help of chemoattractants and integrins)
Polymononuclear cells (PMNs)
the first cells to reach site of injury/infection
typically neutrophils
Mononuclear cells
the second cells to reach the site of injury/infection
typically monocytes and macrophages
Neutrophils
produced in bone marrow and are rapidly recruited to site of injury
function in rapid cytoskeletal rearrangement
migrate through vessel endothelium into the tissue
Macrophages
are recruited more slowly but are more long-lived in the tissue
involved in acute inflammation but are more important for chronic inflammation
Neutrophil function
short-lived cells
5 days in circulation
2-6 hours in tissue
phagocytose and kill bacteria by:
oxygen dependent killing mechanisms
enzymes
death of neutrophils contributes to pus formation and spread of infection
needs to be drained
Systemic changes as a result of inflammation
Leukocytosis - high white blood cell count
increase CSF and bone marrow leukocyte production
Fever
caused by IL-1, TNF, hypothalamus prostaglandin, peripheral vasoconstriction)
Elevated fluid protein
Malaise (discomfort and weakness)
caused by IL-1, TNF, IL-6
Acute phase proteins
serum proteins that increase in concentration by >25% in response to inflammatory cytokines
Fibrinogen (for coagulation)
complement components, C-reactive protein (CRP), Serum amyloid A (SAA) [for anti-bacterial]
Alpha 2 macroglobulin (for proteinase inhibition)
metal metabolism
Sources of pain
Pain receptors are sensitized by:
prostaglandins (PGE2 and PGI2)
Inflammatory mediators act on pain receptors
histamine and bradykinin
Local changes due to inflammation
erythema
swelling
gingival fluid
tooth extrusion
serous crusting
abscess formation
fluid drainage
Outcomes of acute inflammation
Recovery
dilution of toxins by exudate
phagocytosis and destruction of foreign bodies
Treatment
Ulceration (with or without pus formation)
Abscess (necrosis)
Progression to chronic inflammation
Acute pain (nociceptive pain)
Noxious stimuli that bind to nociceptors (pain sensory organs) and threaten or cause tissue damage
Types of nociceptors
Mechanical
Thermal
Polymodal
Silent
Mechanical nociceptor
Located superficially
activated by strong stimuli such as pressure or puncturing of the skin
stimuli transmitted via A-delta fibers
Thermal nociceptors
Located superficially
activated by extreme heat (above 45 C) or extreme cold (below 5 C)
sensation of pain transmitted by A-delta fibers
Polymodal nociceptors
Located throughout the body
activated by noxious mechanical, heat, cold, or irritant (chemical) stimuli
slow, dull pain that persists long after stimulus is removed
transmitted via C fibers
Silent nociceptors
Located in muscle, joints, and viscera
activated by noxious stimuli in muscles, joint, viscera
transmitted via c-fibers
A-delta fibers vs. C-fibers
A-delta fibers: first pain sensations - fast acting - lightly myelinated
C-fibers: second pain response - slow acting - unmyelinated
Difference between nociception and pain
Nociception = transmission of electrical signals from peripheral nociceptors to the CNS
Pain = the perception of unpleasant stimuli by the brain’s nociceptive input. It is a homeostatic mechanism to protect oneself from harm
Pain and nociception are often coupled, but can be experienced independently
Ascending pathway that transmits noxious stimuli
Primary afferent synapses on dorsal horn of second order neurons within spinal cord → ascends to brain for processing
Where do pain and temperature stimuli synapse for the upper limbs
in the cervical spinal cord
Where do pain and temperature stimuli synapse for the lower limbs
in the lumbar spinal cord
Where do pain and temperature stimuli synapse for the orofacial region
Stimuli enter via trigeminal nerve → trigeminal cell bodies in the pons → descend and synapse in spinal trigeminal nucleus in the medulla and upper spinal cord
Spinomesencephalic tract
Neurons start in dorsal horn and ascend to midbrain → crosses on contralateral side to the periaqueductal grey (PAG) which surrounds the cerebral aqueduct
PAG mediates fight or flight
response and modulates pain stimuli
Spinoreticular tract
from dorsal horn of spinal cord to reticular formation of brainstem
senses temperature, pressure, and visual/auditory information
Spinothalmic tract
from dorsal horn of spinal cord to the thalamus and the to various parts of cerebral cortex
provides sensory and emotional information that are integrated in the prefrontal cortex, insular cortex, cingular gyrus (not as important in survival instinct)
Pain modulating pathway
Numbs the brain to pain in response to overwhelming noxious stimuli via endogenous analgesia
endogenous analgesia begins in PAG of midbrain → medulla → spinal cord → trigeminal nucleus
Opioids act in same way
Lewis triple response
Characterizes physiological pain
retrograde activation of C-fibers releases Substance P which sensitizes nociceptors
Substance P triggers mast cell degradation and histamine release = swelling
Tissue damage = local release of bradykinin, prostaglandins, K+ which bind to nociceptors
Primary hyperalgesia/allodynia
sensitization of nociceptors leading to increased pain response to mechanical stimuli
Allodynia = previous innocuous (non-painful) stimuli becomes painful
Hyperalgesia = noxious stimuli perceived as more noxious
Central sensitization (normal acute pain)
CNS becomes more sensitive to pain as a result of glutamate (rapid depolarization of second order neurons) and substance P (delayed, long-lasting depolarization) signals
Glutamate binds to AMPA
Substance P binds to neurokinin1
Persistent pain response
Persistent pain in spinal trigeminal neurons open the NMDA receptor (normally blocked by Mg) → causes Ca and Mg release that increase pain response → secondary hyperalgesia develops to increase and prolong pain
Pain may be modulated via Opioids, adrenergic agents, GABA, 5HT receptors
Nitric oxide
product of the opening of the NMDA receptor → releases Ca
prolonged activity of spinal trigeminal neuron triggers death of spinal trigeminal cells → intracellular Ca → production of nitric oxide synthase
Nitric oxide is toxic to pain inhibitory neurons (GABA) and contributes to increase pain experience
Types of cells in the nervous system
Neurons (usually multipolar)
Glia
Classifications of Glia
CNS
Astrocytes
Microglia
Oligodendrocytes
Ependymal
PNS
Schwann cells
Astrocytes
neuron support, BBB maintenance, injury response
Microglia
immune cells, inflammation, injury response, synaptic pruning
Oligodendrocyte
myelination
Ependymal
CSF secretion
Schwann Cells
myelination, neuro-regeneration
Multipolar cells
one axon extends from cell body with multiple dendrites, facilitating communication with other neurons
Bipolar Cells
single dendrite and one axon on other side
Unipolar/Pseudounipolar cells
Cell body in the middle of two axons - one axon innervates dendrites and the other travels to CNS
Myelinated vs Unmyelinated cells
Myelinated
fast impulse transmission via saltatory conduction
contains Nodes of Ranvier
Unmyelinated
slower impulse transmission with continuous conduction
responsible for dull aches and burning sensations
Hydropic change
cellular injury characterized by a larger than normal vacuole in the cell
Fatty change
cellular injury characterized by increase lipid vacuoles in the cell
Hypertrophy, Hypotrophy, Atrophy
Hypertrophy = increase in size
Hypotrophy = decrease in size
Atrophy = death of tissue
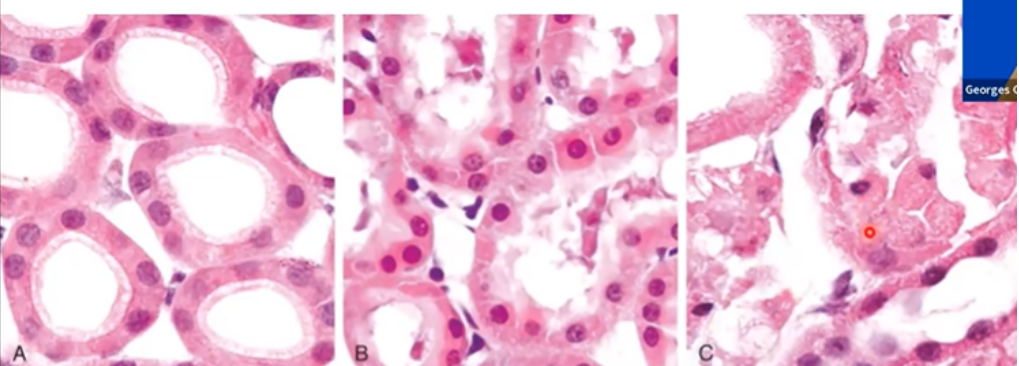
Describe the injury in this image (A, B, C)
Kidney Tubules Hydropic Degeneration
A) Normal
B) Early reversible ischaemic injury - increased eosinophilia of cytoplasm - protein denatured - swelling
C) Necrotic irreversible injury - loss of nucleus and cell fragmentation
Cardiac muscle Hypertrophy
causes myocytes to become bigger
continue growth will injure myocytes → cell death
contribute to acute myocardial infarction
Sialosis
cellular injury characterized by enlargement (hypertrophy) or reduction (atrophy) of acinar cells of the major salivary glands
Lipofuscin
a yellow-brown pigment made of lipid-phospholipid-protein polymers
contributes to oxidative stress of organelle membranes
Commonly known as “wear and tear pigment”
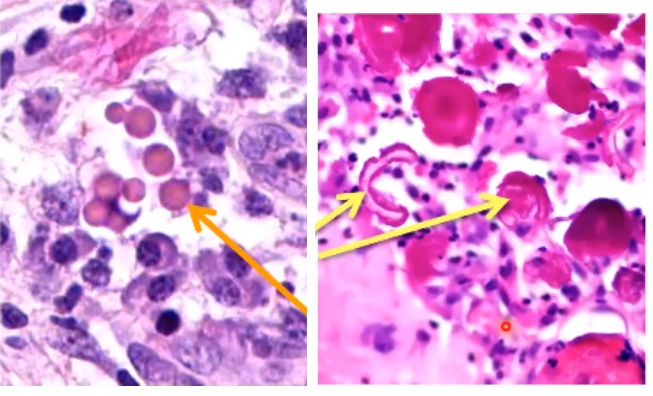
Hyaline change
Left: Russel bodies
found in plasma cells
excessive immunoglobulin synthesis
Right: Rushton’s bodies
specific to odontogenic cysts
found in the epithelium of cysts
Hyperplasia and example
Excessive cell proliferation
Ex. Traumatic Keratosis - epithelial cells proliferating to form increase cells on surface
Metaplasia and example
change in cell differentiation from one form to another
Ex. Smoker’s lung epithelium will shift to become squamous to protect airways
Dysplasia and example
disordered growth that alters size, shape, and organization of cells in tissue
reversible
may be cancerous
Ex. cancer of skin epithelium, bronchus, cervix
Anaplasia
loss of differentiation only in presence of malignant neoplasia (cells that become cancerous)
Cell Death (Coagulation)
Death of nucleus while cell remains intact
Types:
pyknosis - nuclear shrinkage
karyorrhexis - nuclear fragmentation
karyolysis - nuclear degradation
Cell Death (Liquefaction)
Associated with microbial infection
Cell becomes soft, liquefied and filled with pus
Cell death (Caseous)
Cheese like appearance under a microscope
Symptoms of coagulation and liquefaction
Caused by tuberculosis
Cell death (Fat necrosis)
Occurs in fat cells
Enzymes break down fats leaving free fatty acids in cell
Fatty acids react with calcium to form deposits
Often occurs in pancreas and breasts
Common structures that arise from apoptosis
fusion of palate, gland lumen formation, involution of breast tissue
Pathological apoptosis
death of healing cells, atherosclerosis, autoimmune disease
Caused by:
radiation, toxins, free radical → DNA damage
withdrawal of growth factors/hormones
receptor-ligand mediated apoptosis
Cytotoxic T cells triggering apoptosis
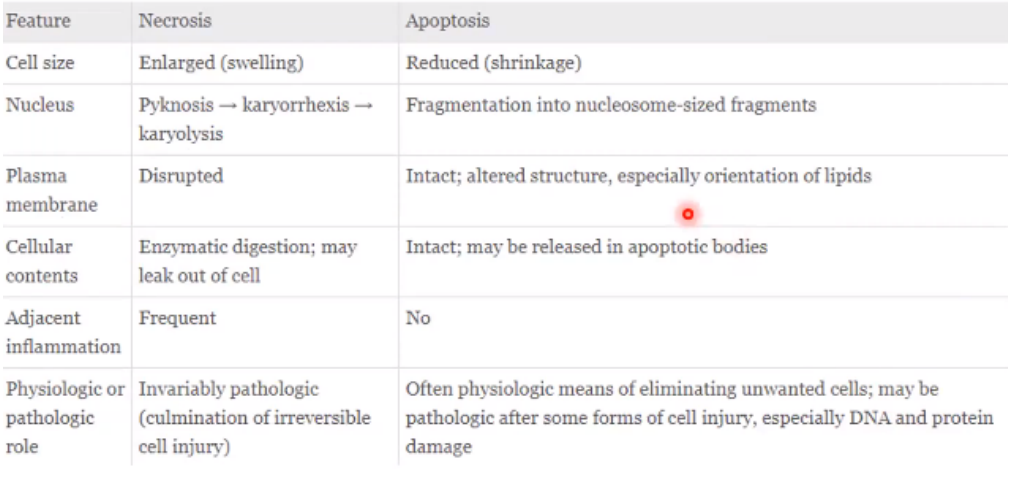
Necrosis vs. Apoptosis
Processes that cause Cell Death
ATP depletion
loss of energy-dependent cell functions = cell death
Loss of membrane integrity
Mitochondria membrane disruption = loss of ATP and cell death
Lysosomes can degrade cell membrane = death
Plasma membrane degradation = loss of cell contents and death
Increase intracellular Ca
Protein breakdown and DNA damage = cell death
Reactive oxidative stress
Protein breakdown and DNA damage = cell death
Dental Pulp
Soft connective tissue core of tooth
Contains:
cells
water
collagen
blood vessels
lymphatics
nerves
Functions of dental pulp
nourish the dentine of the tooth via blood vessels
provide sensation (temp, pressure, pain)
protection (immune tissue through lymphatic system and production of tertiary dentine)
The pulp chamber
Coronal Pulp = pulp closest to the crown
Radicular Pulp = pulp in the root canals
Pulp chamber surrounded by periodontium
Pulp chamber may have accessory canals
canals can be potential avenue for bacterial passage
Dentine-pulp complex
odontoblasts present at dentine-pulp junction on pulpal side
produce and secrete dentine
have processes that extend into dentine via dentinal tubules
Stimulus/injury of either causes response from other
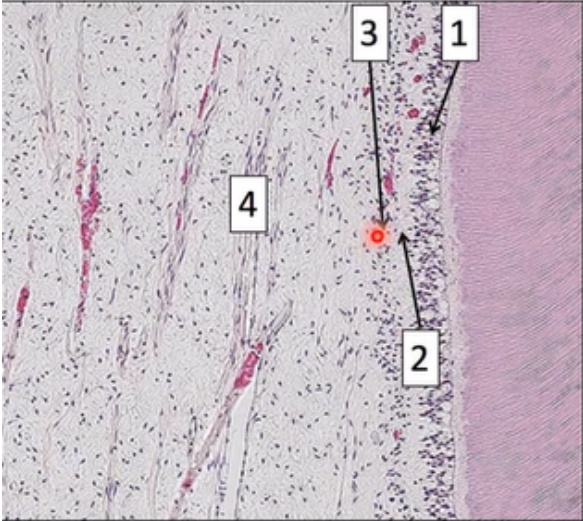
Histological zones of pulp
Odontoblastic zone
Cell-free zone of Weil
Cell-rich zone
Pulp core
Odontoblastic zone
consists of the cell bodies of the odontoblasts
dentinal tubules linked together laterally via tight junctions and adherens junctions
serum protein can pass between these junctions
lifespan of odontoblasts in this zone linked to lifespan of tooth
Cell-free Zone of Weil
region with very few cell bodies and nuclei
evident in coronal pulp
not present during development
contains capillaries, nerves
called Subodontoblastic plexus of Raschkow
Cell-rich Zone
region of high cell-density
most evident in coronal pulp
many fibroblasts and stem cells (become odontoblasts)
Pulp core
majority of pulp volume
contains major vessels, nerves, and connective tissue matrix
CT matrix = fibroblasts, immune cells, undifferentiated mesenchymal cells, odontoclasts
Innervation of pulp
nerves (sensory and sympathetic) enter pulp via apical foramen
nerve bundles run along blood vessels and branch out toward periphery of the pulp
these nerves contribute to nerves in cell-free zone (subodontoblastic plexus of Raschkow)
Drug definition
substance that exerts biological effect when administered to the body
may be natural or synthetic
Medicine definition
preparation of substances to be administered for therapeutic use
Process of medicine approval
Pre-clinical testing → clinical trial → licensing approval → patient access
Pharmacology
the study of drugs used in therapeutics
Pharmacotherapeutics
application of pharmacological knowledge to use on drugs to manage a condition
Pharmacokinetics
what the body does to the drug (absorption, distribution, metabolism, excretion)
Pharmacodynamics
what the drug does to the body
Clinical pharmacology
right medicine + patient + dose + dose form + route + time + documentation = right response
Regulatory bodies of medicines in Australia
Therapeutic Good Administration (TGA)
Quality us of Medicines (QUM)
National Medicines Policy
Common medicines in Australia for cardiac conditions
rovuvastatin
atorvastatin
pantoprazole
Resilient Arteries Protected (RAP)
Nurofen
Drug Class:
NSAID, analgesic, antipyretic
Mechanism of Action:
inhibits cyclooxygenase 1 and 2, decrease prostaglandins
Indications (what it treats):
pain, inflammation, arthritis, fever
Precautions:
dehydration, asthma, coagulation disorders, renal impairment, cardiovas disease, GI issues, elderly, pregnany
Contraindications:
active peptic ulcer or GI bleeding
Adverse Effects:
nausea, salt/fluid retention, hypertension, diarrhea, stroke, heart attack, rash
Dose:
200-400mg (1-2 tabs), 3-4 times daily