Chemistry 10th Honors- Midterm
1/53
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
54 Terms
What is the current model of the atom?
Schrondinger
Name the Model:
-Electron Cloud Model
-Electrons are constantly in motion around the nucleus in different shaped orbitals
-Electrons have properties of waves and particles
-Nucleus contains protons and neutrons
Schrodinger
Who proposed the existence of the atom?
Democritus
Who said atoms are solid, homogeneous, indestructible, and indivisible?
Democritus
Who said atoms have different shapes and sizes?
Democritus
Who rejected the ideas of Democritus?
Aristotle
Who said empty space cannot exist?
Aristotle
Who said matter is made of earth, fire, air, and water?
Aristotle
Who said atoms are building blocks of matter?
John Dalton
Who said atoms are indivisible?
John Dalton
Who said atoms of the same element are identical?
John Dalton
Who said atoms cannot be created or destroyed, only rearranged in chemical reactions?
John Dalton
Who said atoms unite in small, whole number ratios to form compounds?
John Dalton
Who said there was a Solid sphere model (Billiard Ball)?
John Dalton
Who discovered the electron (proved Dalton’s indivisible atom idea was wrong)?
J.J. Thomson
Who proposed the plum pudding model?
J.J. Thomson
What is the Plum Pudding Model?
Model that suggests:
Negatively-charged “plums” in a positively-charged pudding
Who said most of the atom is empty space?
Ernest Rutherford
Who said atoms have a dense, positively-charged center?
Ernest Rutherford
What is the name of Ernest Rutherford’s experiment?
Gold-foil experiment
What did Rutherford do in the Gold Foil experiment?
Aimed alpha particles at gold foil
Most particles passed through,
but some bounced back
What did the Gold Foil Experiment prove?
the existence of a dense center as opposed to a positive blob with electrons stuck to it
Who worked with Rutherford?
Niels Bohr
Who kept the idea of the positive center and said electrons are in energy levels and orbit the nucleus like planets around the sun?
Niels Bohr
Who proposed the planetary model?
Niels Bohr
Who built off Bohr’s model?
James Chadwick
Who discovered the neutron?
James Chadwick
Who said the mass of a neutron = Mass of a proton
and the neutron is neutral?
James Chadwick
Put the atom scientists in order.
Democritus→Aristotle→John Dalton→J.J. Thompson→Ernest Rutherford→Niels Bohr→James Chadwick
A mass spectrum of Argon shows that it has three major isotopes, Argon-36 (0.34%), Argon-38 (0.06%), and Argon-40 (99.60%).
Which of the following best explains why the average atomic mass of Argon is 39.948 amu?
The average is closest to Argon-40 because it is found in the highest percentage.
Using correct significant figure rules, what would be the average atomic mass of two isotopes of an unknown element. One isotope is 14.00 amu and 99.6%, and the other is 15.00 amu and 0.4%.
14.0 amu
There are two isotopes of an unknown element. One has a mass of 10.01 amu and a percentage of 19.1%. The other has a mass of 11.01 amu and a percentage of 80.9%. What is the average atomic mass of this unknown element?
10.82 amu
When using the percent abundance of each isotope of an element to calculate average atomic mass, it is important to remember to...
convert it back to decimal form
What two things are needed to calculate average atomic mass?
Mass # and percent abundance
There are four major isotopes of Cerium: Cerium-136, Cerium-138, Cerium-140, and Cerium-142. One of these isotopes has an abundance of 88.4%, one 11.1%, and the remaining two are 0.2% and 0.3%.
If the average atomic mass of Cerium is 140.116 amu, which isotope is the most abundant?
Cerium-140
What is the mass number of Be with 5 neutrons?
9
The number of protons is equal to the number of ________.
electrons
What is the mass number of Ga with 41 neutrons?
72
What is the atomic number of Bromine-79?
35
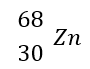
What is the atomic number?
30
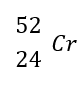
What is the mass number?
52
Neutral atoms have no electrical charge because they
have an equal number of electrons and neutrons
Atoms with the same number of protons but different numbers of neutrons
isotopes
All of the elements in group 2/2A will have the following charge:
+2
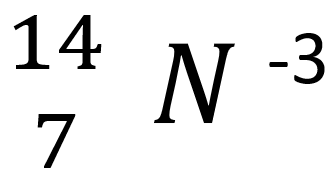
How many electrons does the following element have?
10