hydrocarbons and fractional distillation
1/13
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
14 Terms
What is crude (unrefined) oil ?
it’s a complex mixture of hydrocarbons with differing number of carbons (diff chain lengths and rings)
an important source of useful substances (fuels and feedstock for the petrochemical industry)
a finite resource
How is crude oiled useful ?
it’s useful for fuel
as a base material (feedstock) for the petrochemical industry
it’s also a finite resource and will run out
What is a hydrocarbon ?
a compound that ONLY contains hydrogen and carbon
Draw the process of fractional distillation ?
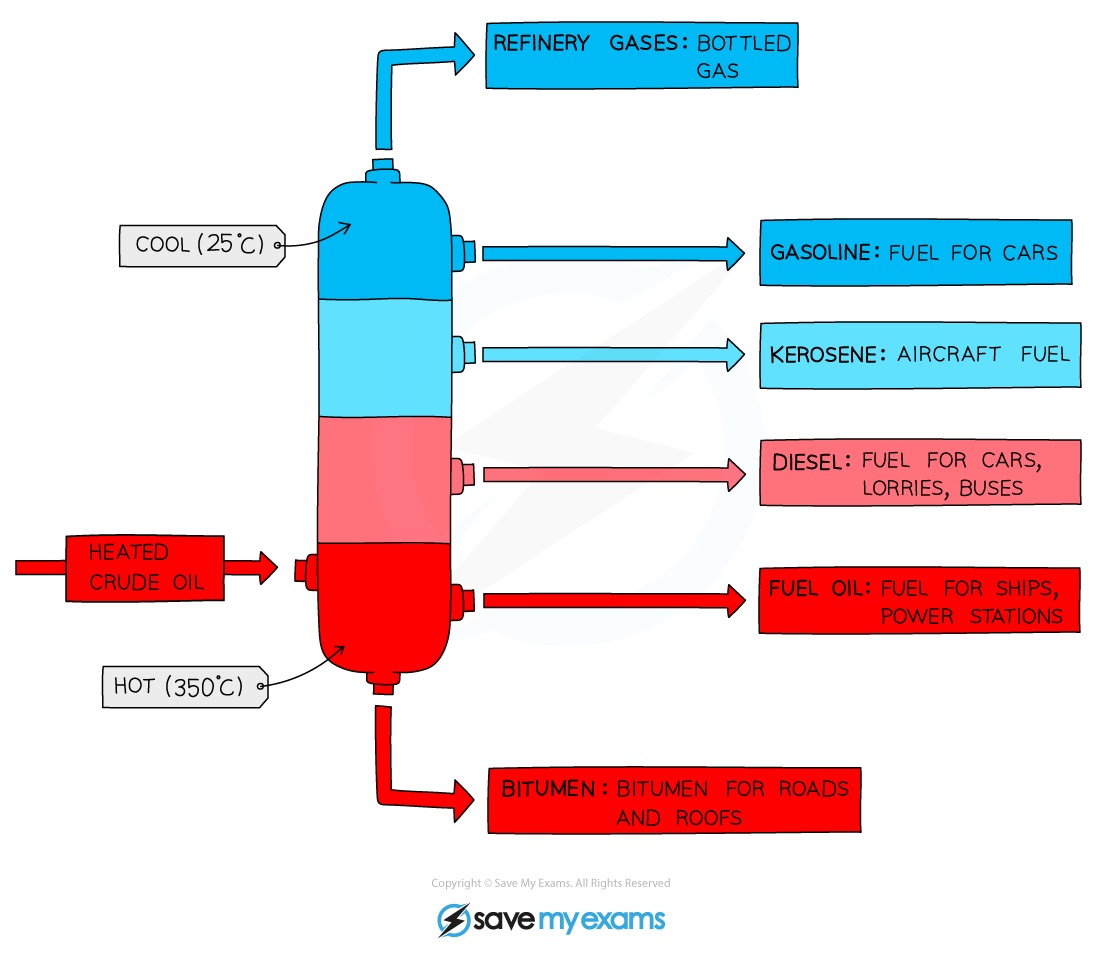
What is bitumen used for ?
surfacing rods and roofs
What is fuel oil used for ?
fuel for large ships and power stations
What is diesel oil used for ?
fuel for some cars and trains
What is kerosene used for ?
fuel for aircraft
What is petrol used for ?
fuel for cars
What is gases used for ?
domestic heating and cooking
In the fractioning column, where is the hottest and coldest ?
hottest at the bottom
coldest at the top
In the fractioning column, what happens to the vapours ?
vapours rise through column and cool down
they condense when they reach a part of the column that is cool enough
liquid falls into a tray and is piped away
The longer the hydrocarbon……?
the thicker it is