Quality Assurance
1/37
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
38 Terms
Quality Assurance
a broad spectrum of plans, policies and procedures that together provide an administrative structure for a laboratory's efforts to achieve accurate results
elements of a Quality Assurance program
1. Commitment of the Institution/Department
2. Facilities and Resources
3. Technical Competence
4. Problem-Solving Mechanism
Commitment to the Institution/Department
dedication to quality service must be central;
quality must be a major consideration in all management decisions
Facilities and Resources
laboratories must have adequate space, equipment, materials, supplies, staff and supervisory personnel and budgetary resources
Technical Competence
high quality personnel are essential for high quality service
Problem-Solving Mechanism
The laboratory must establish policies and procedures to monitor and assess, and when indicated, correct problems identified
this also includes review of the effectiveness of corrective actions taken to resolve and monitor problems
types of variables evaluated by Quality Assurance
1. pre-analytical
2. analytical
3. post-analytical
types of variables evaluated by Quality Control
1. analytical only
pre-analytical variables
test requests,
patient preparation and identification,
specimen acquisition and transport,
specimen processing and distribution,
preparation of worklists and logs,
maintenance of records
analytical variables
procedures and methods used for tests performed,
documentation of testing protocols and procedures,
monitoring of critical equipment and materials
post-analytical variables
reporting of results;
critical values
how Analytical Quality is monitored
statistical methods (QC)
quality control makeup
the use of commercially prepared biological material;
this material should be of the same makeup as the actual patient specimens being tested;
e.g. for a serum test, should purchase a serum-like substance
quality control precautions
because of its makeup, could be potentially infectious and should be treated with the same standard precautions that are used for actual patient specimens
how quality control is used
it has a 'known' value for each analyte, but it is treated as an 'unknown' sample when unknown patient samples are being analyzed
why quality control is used
to ensure the reliability of each measurement performed on a patient sample
evaluation of quality control
compare the QC test result to the known QC range for the respective QC material;
if the QC result is not in this range, patient results cannot be reported until this problem is investigated
establishing known range for quality control
a calculation performed by testing the QC material 20 times,
then calculating the mean plus or minus 2 SD
types of error detected by quality control
only detects analytical errors
- systematic analytical errors
- random analytical errors
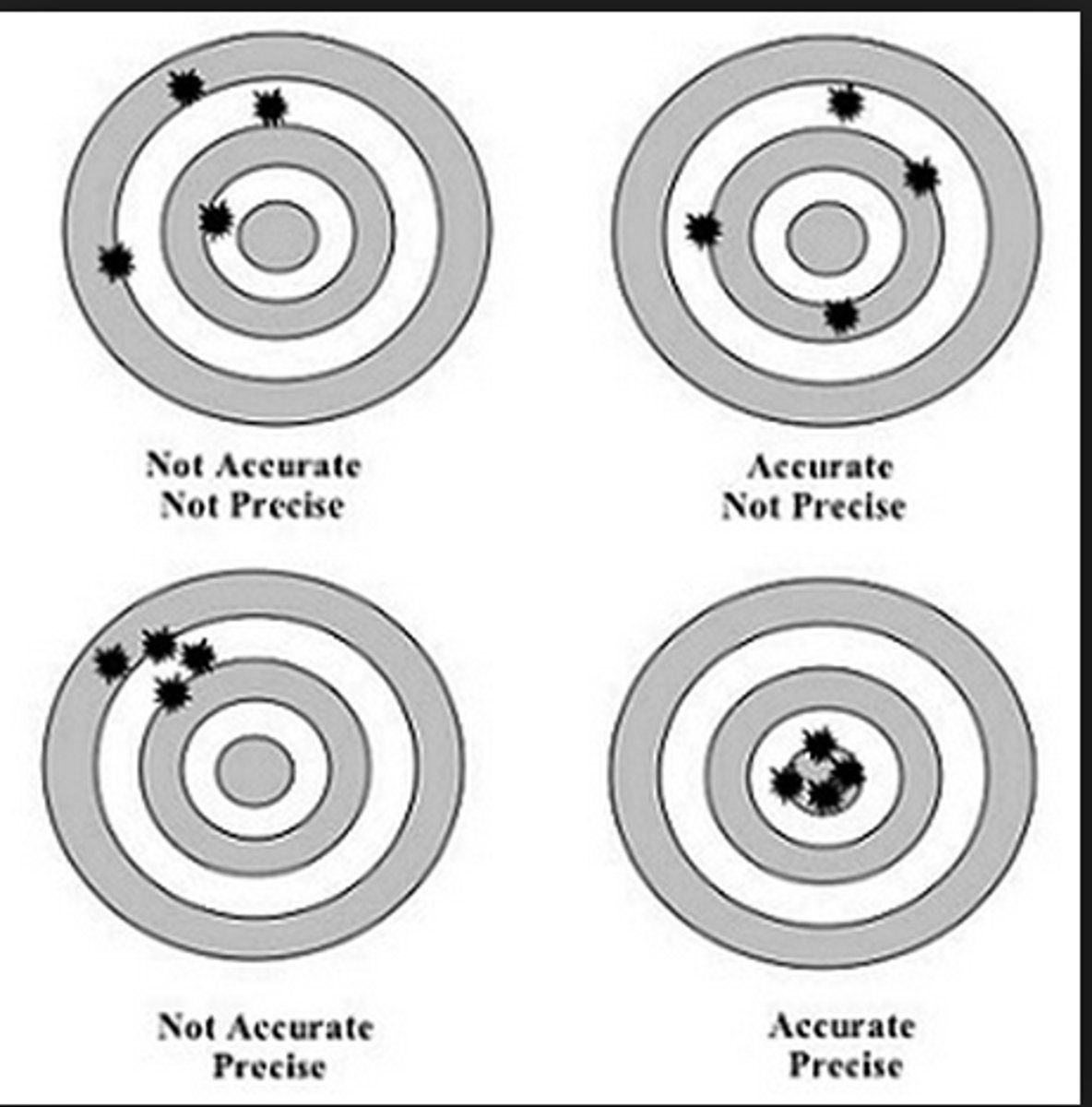
accuracy
the relative exactness by which a value represents the true value;
a change in accuracy is reflected by a change in the mean
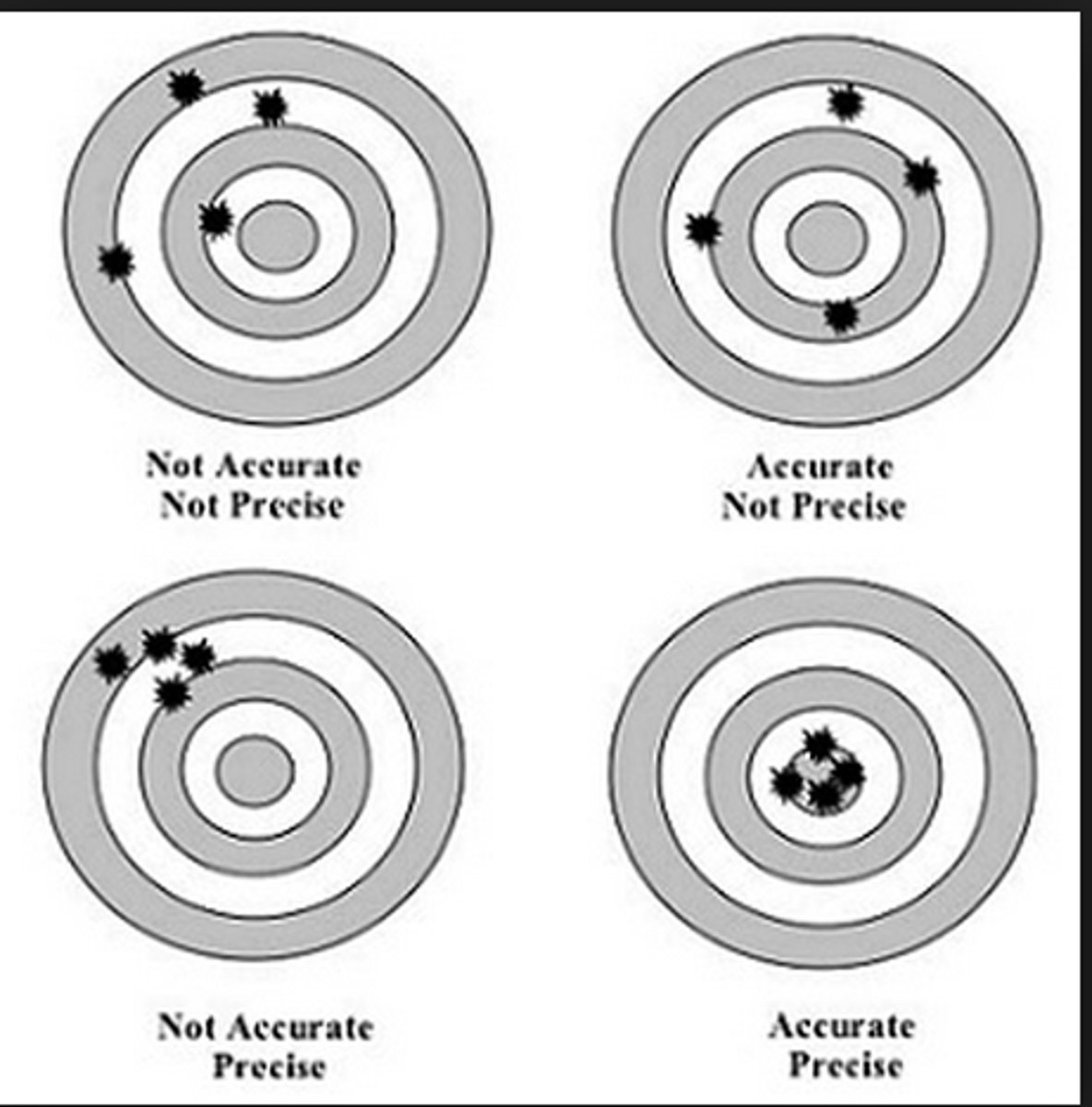
precision
the relationship between replicate results and how closely they group together;
a change in precision is reflected by a change in reproducibility / degree of variation
2 calculations:
standard deviation
coefficient of variation
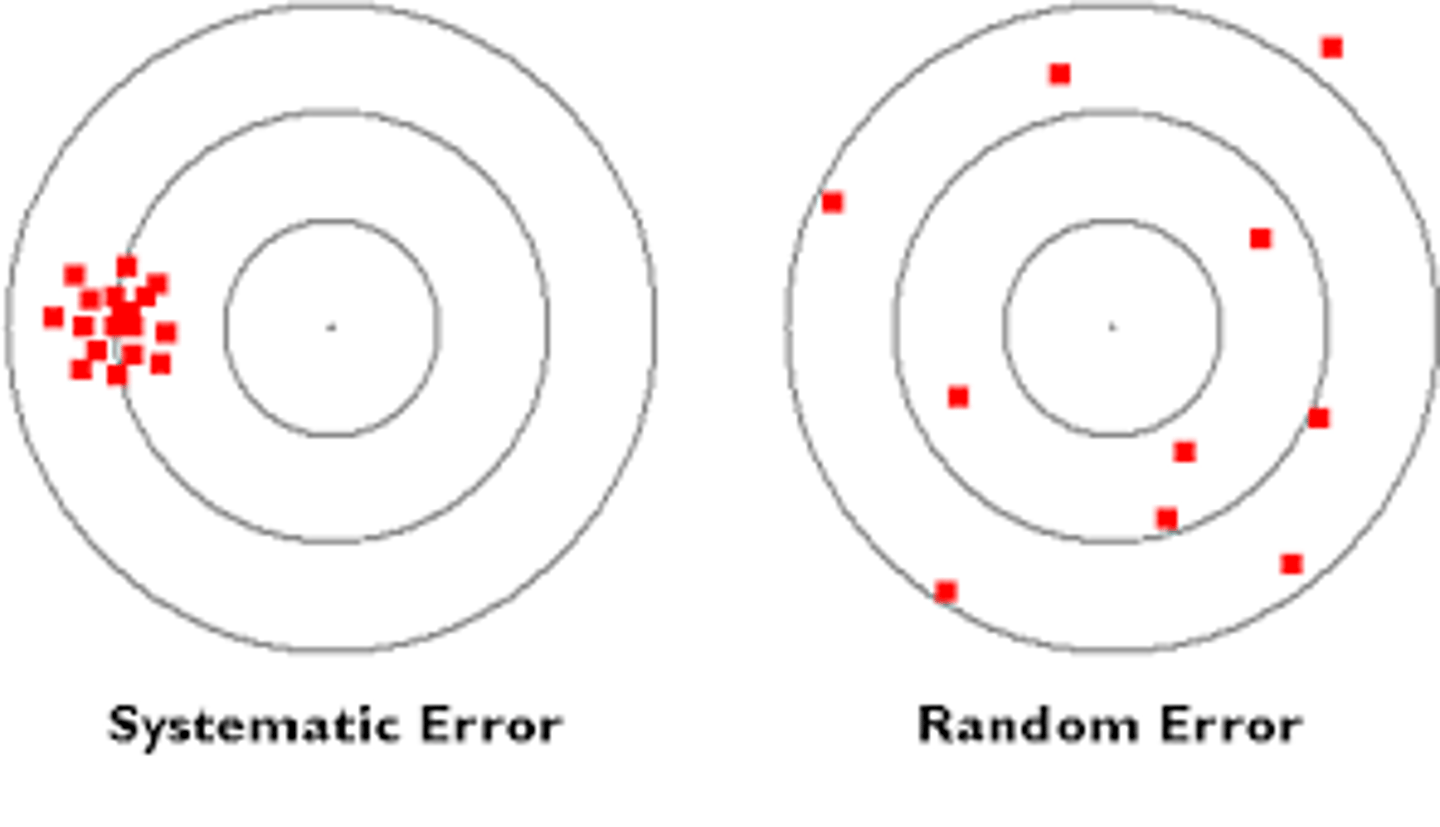
systematic error
errors in accuracy;
predictable variation;
where the instrument/method is making the same error every time the test is run;
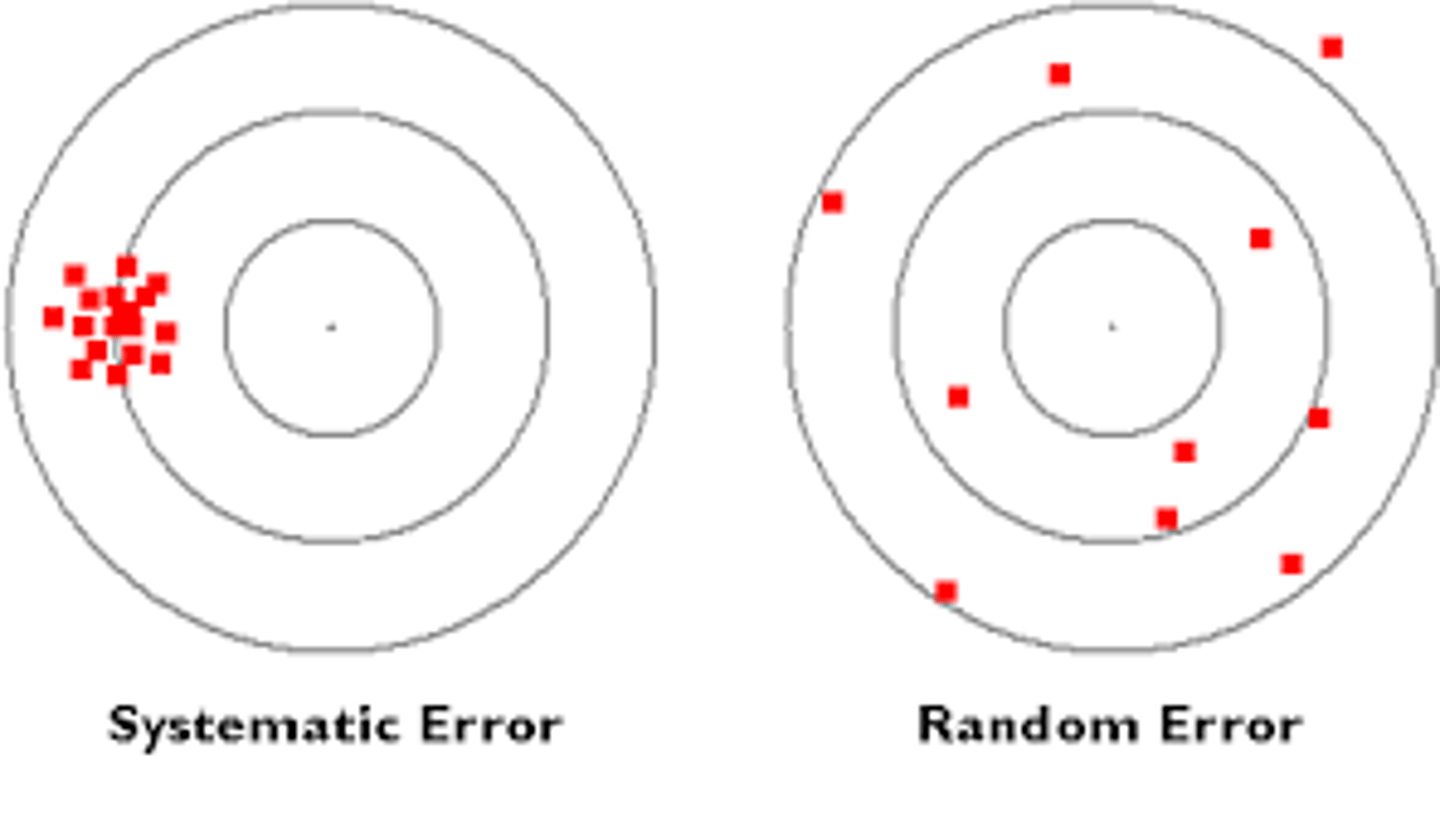
random error
errors in precision;
unpredictable variation;
problems associated with reproducing test results;
analytical errors/problems associated with systematic variation
impure / contaminated calibration standards or reagents,
inadequate calibration techniques,
improper calibration standard or reagent preparation
analytical errors/problems associated with random variation
pipetting samples and reagents,
mixing samples and reagents,
instrument instability,
temperature / timing instability
outlier
a result(s) lying outside the acceptable QC range
(mean +/- 2SD)
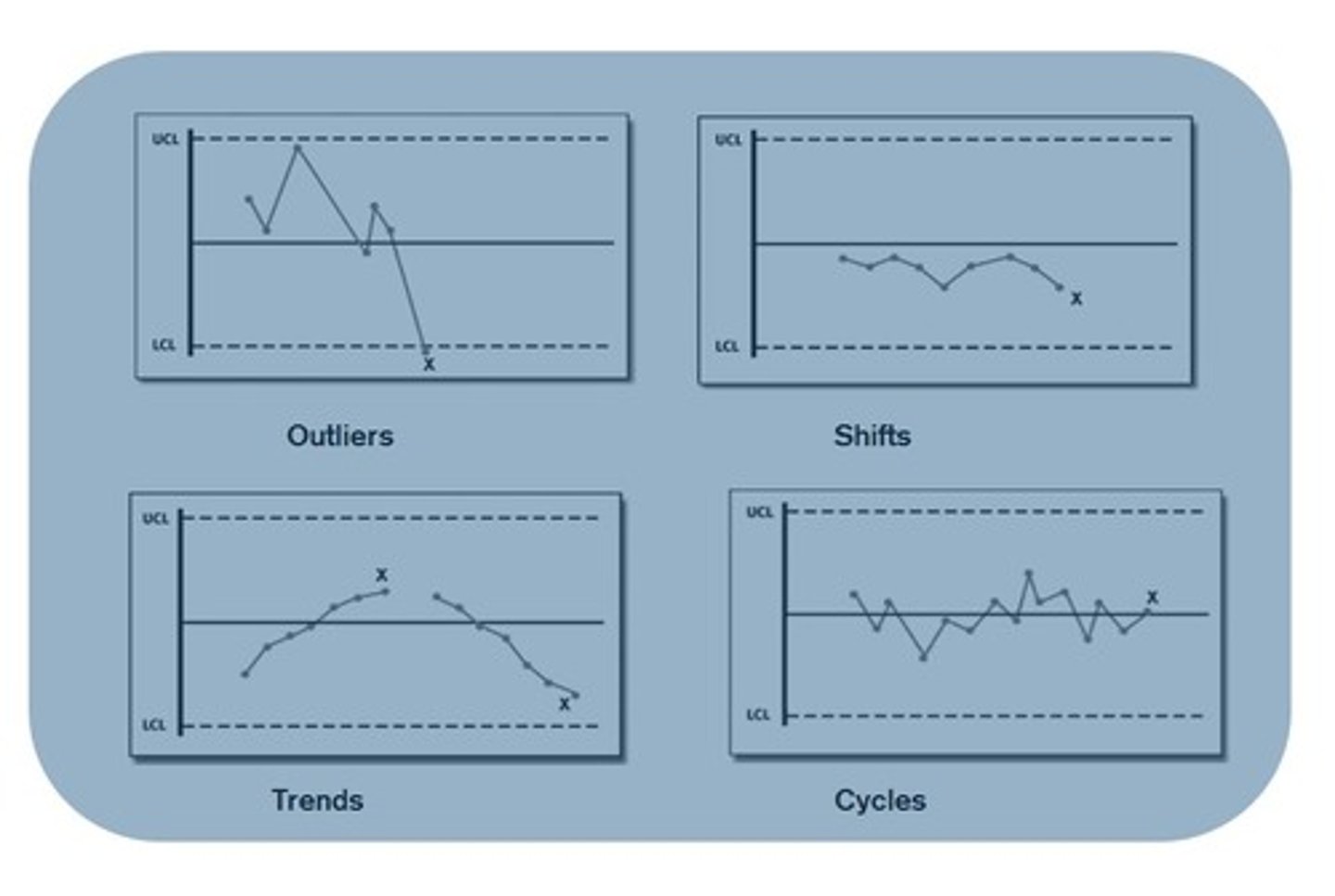
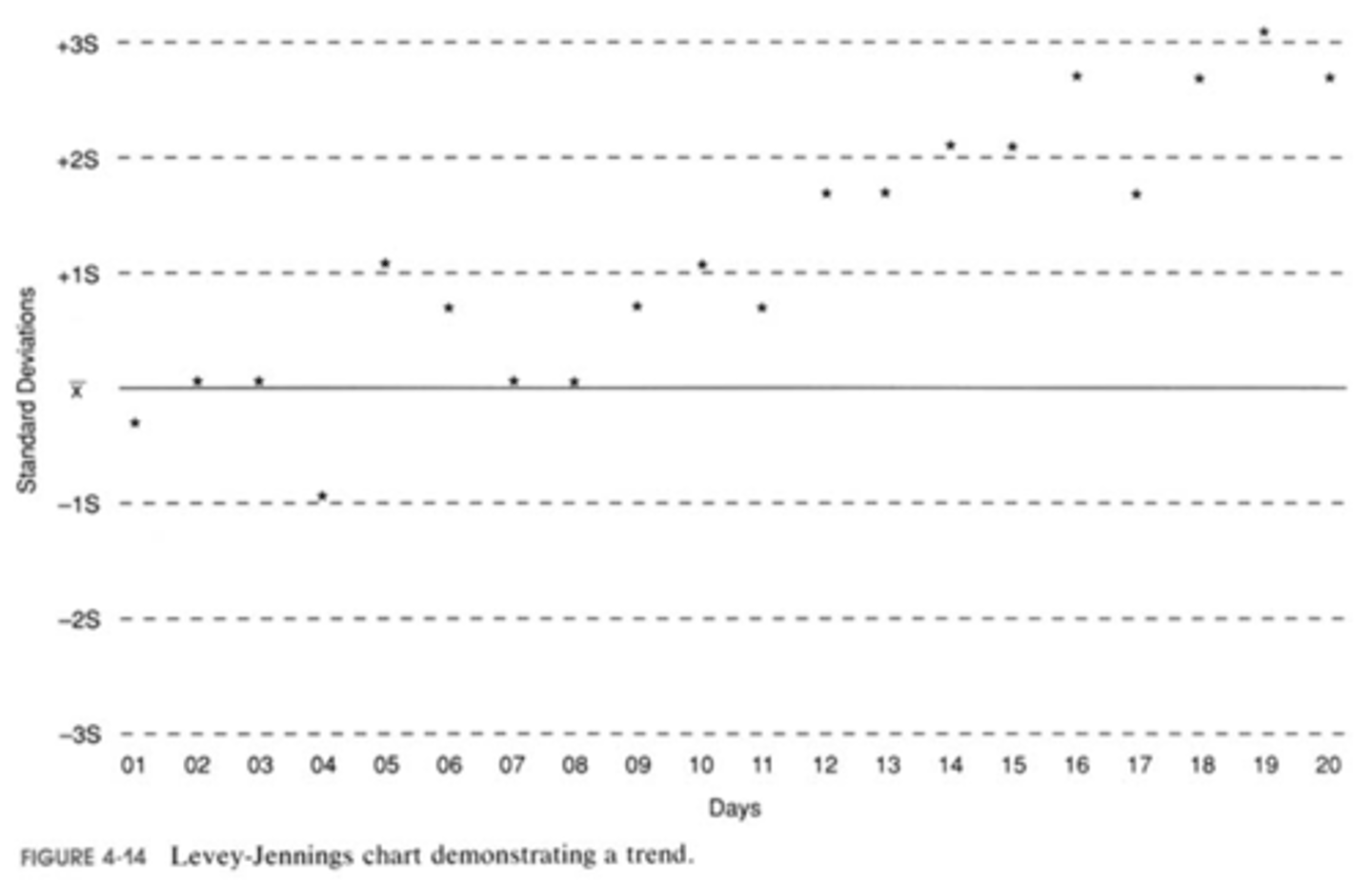
trend
a gradual change in one direction of the result away from the mean;
possible causes:
slow deterioration of reagents,
slow change in calibration standards,
slow failure of an instrument component
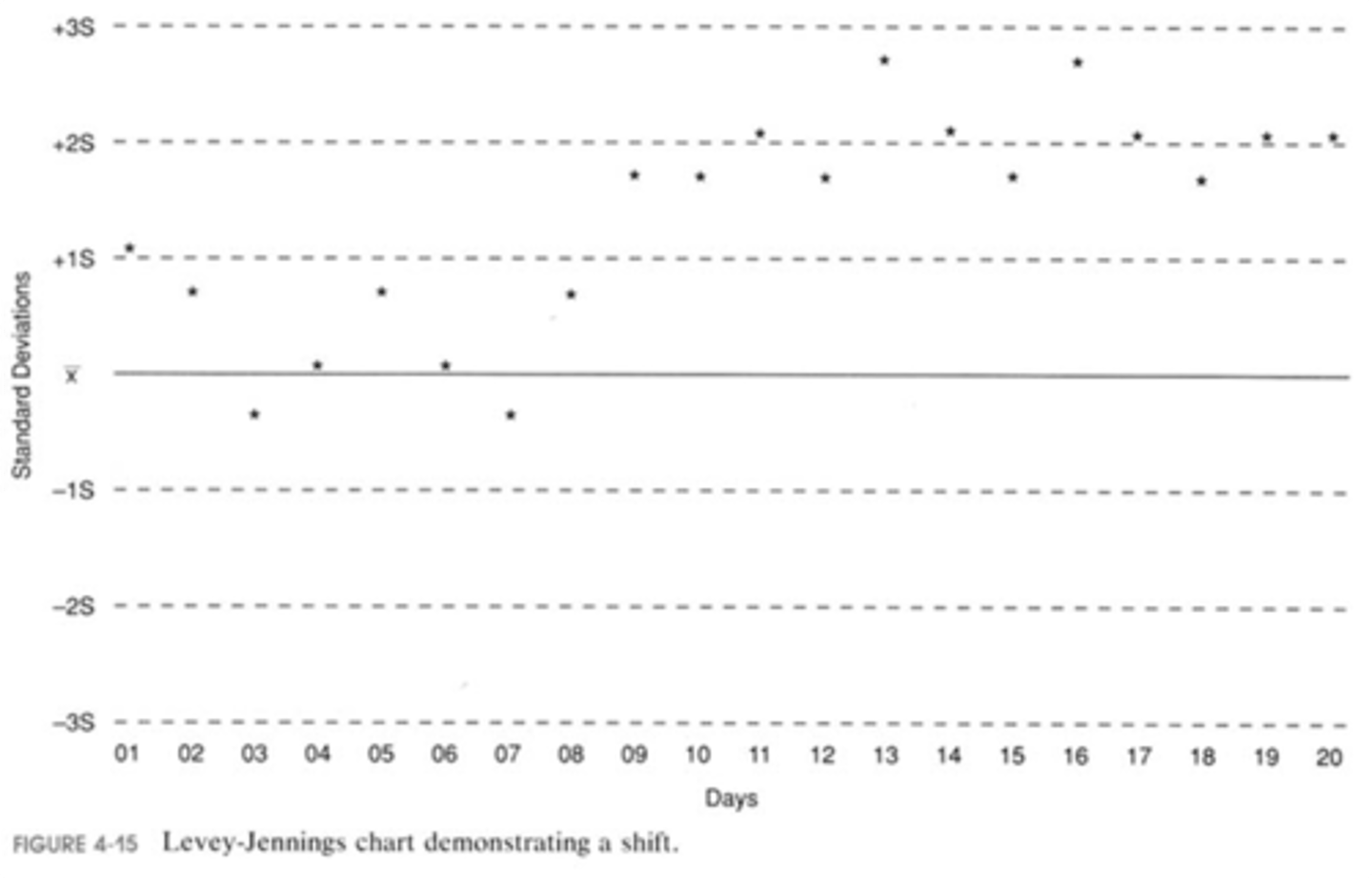
shift
an abrupt change in an analytical system that continues at the new level;
possible causes:
changed to a new lot of reagent,
changed to a new lot of calibration standard,
sudden change in instrument sensitivity
why quality control errors should be investigated
patient data is influenced in the same way as QC material
types of error that cannot be detected by quality control
1. Inter-individual variations
2. Intra-individual variations
3. Pre-analytical errors
4. Post-analytical errors
Inter-individual variations
between individuals;
variations due to age, gender, race and genetic differences
Intra-individual variations
same individual;
variation due to emotional states, activity, diet, drugs, sleep, posture, etc.
Pre-analytical errors
errors due to specimen handling (collection, transport, centrifugation, storage, etc.);
wrong patient drawn,
collection error,
transport / processing error,
storage error
Post-analytical errors
variations due to recording and transmission of patient data to the physician;
reading, transcribing, reporting errors
delta check
checking the 'new' test result with the 'previous' patient test result from a previous time or day (if available)
Compare and Contrast Quality Assurance & Quality Control, including:(1) Which is a broad scope and which is specific(2) What categories of testing fall under QA? QC?(3) What is the desired outcome for both QA and QC?(4) Name 3 components included in QA
Quality Assurance is broad scope, and Quality Control is specific. Quality Assurance evaluates Pre-Analytical, Analytical and Post-Analytical procedures. Quality Control evaluates only Analytical Procedures. The desired outcome of both QA and QC is to produce accurate results. Components included in QA include (know 3): Commitment of the Institution, Adequate Facilities and Resources (space, equipment, materials, supplies staff, supervisory personnel, budget), Competent personnel, Trouble-shooting/Problem-solving (monitor, assess and correct problems identified).
Describe how QC can detect Analytical variations/errors, including:(1) How QC catches a change in Accuracy vs. Precision(2) What types of error changes in Accuracy and Precision are representative of(3) Provide one example of what could happen to produce each of these types of error
A change in accuracy is reflected by a change in the mean, while a change in precision is reflected by a change in reproducibility / standard deviation. Changes in accuracy often represent Systematic Errors, while changes in precision often represent Random Errors. A Systematic Error that can cause a change in Accuracy could be (know one): Contaminated Standards or Reagents, Improper Calibration, Improperly prepared Standards or Reagents. A Random Error that can cause a change in Precision could be (know one): Pipetting variability, Mixing variability, Instrument instability, Temperature instability.
Explain the Westgard Rules, including:(1) When are the Westgard Rules used in the lab?(2) What do Westgard Rules help to detect?(3) Be able to evaluate a set of data and apply Westgard Rules, including naming the Westgard Rule that applies, briefly describing how you identified that Westgard Rule, and what needs to happen next (i.e. warning, rejection, recalibrate)
The Westgard Rules are used during the evaluation of QC, to detect whether there are Random or Systemic Errors in the test method. In the data set included, the Westgard Rule violated is _____. The reason this rule is violated is _______. When this Westgard Rule is violated _______ (QC can be accepted as it is just a warning / This QC run must be rejected / This test should be recalibrated, etc.)