Quiz Chapter 13 alkenes alkynes aromatics
1/22
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
23 Terms
The term used to describe the geometry of a carbon atom involved in a double bond is
trigonal planar.
The name of the polymer formed from CH2=CH2 is
polyethylene
The bond angle about a carbon atom involved in a triple bond is
180 deegres
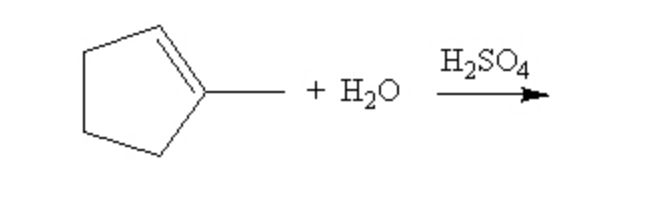
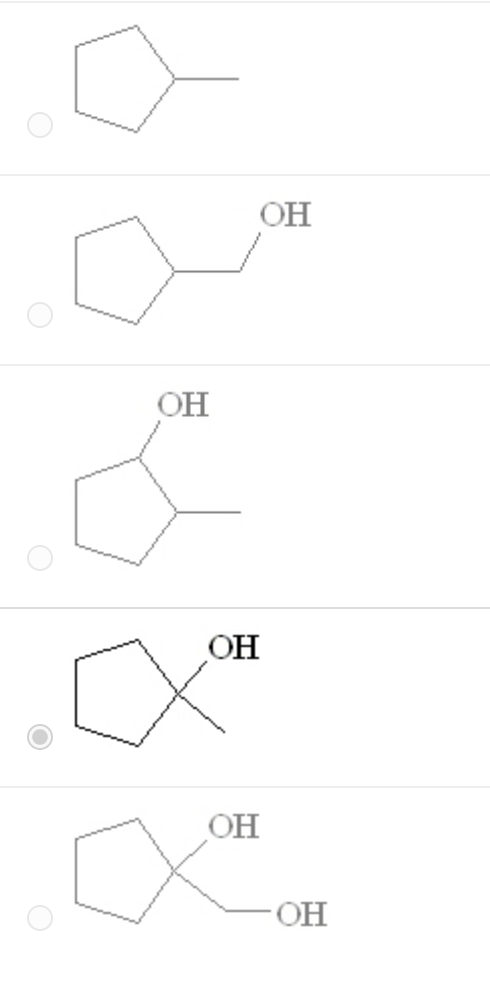
Predict the product of the following reaction:
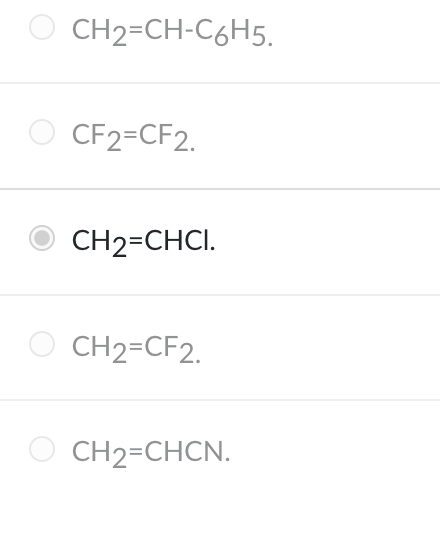
The monomer used to make the polymer polyvinyl chloride is
CH2=CHCl.
According to Markovnikov's rule, when HCl reacts with the molecule shown, which product will result?
(CH3)2C=CHCH3 + HCl → ?????
(CH3)2CClCH2CH3

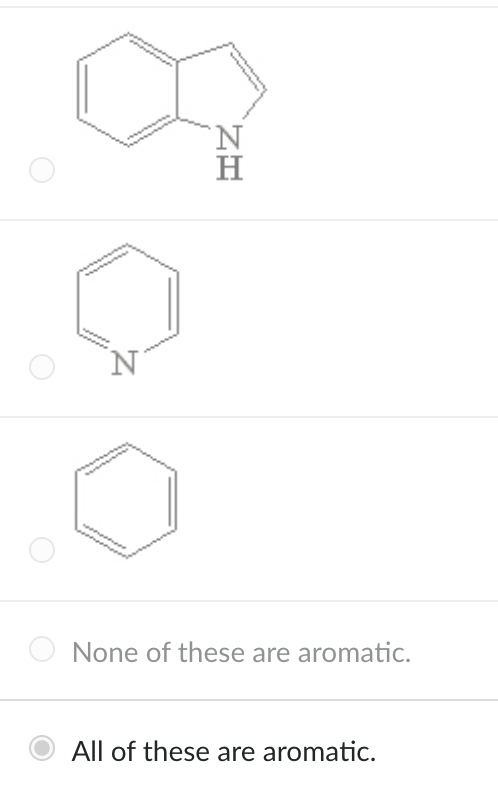
Which of the following is(are) aromatic compounds?
all of these are correct
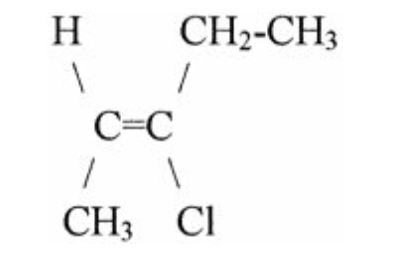
The name of the molecule shown is
trans-3-chloro-2-pentene.
Which of the following compounds contains an alkene functional group?
CH3 CH2CH=C(Ch3)2
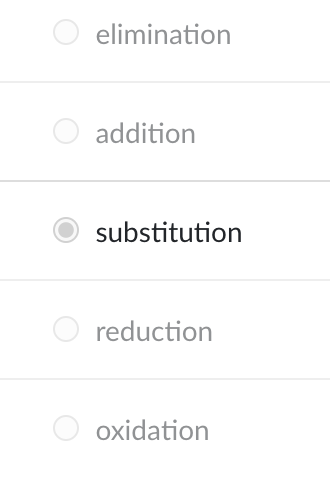
The most common reactions involving aromatics are ________ reactions.
substitution
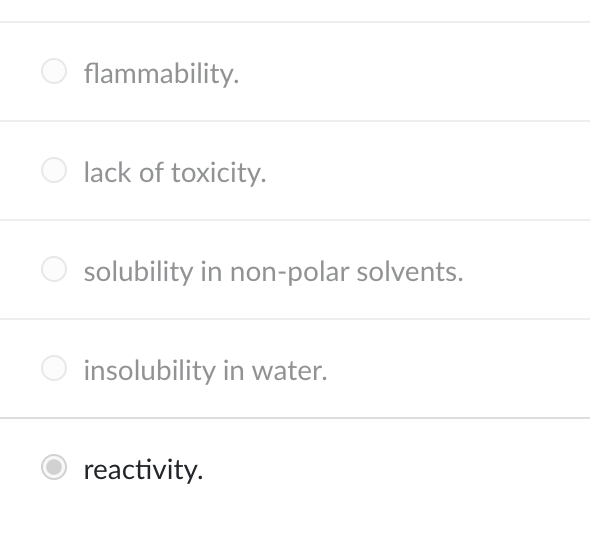
Alkanes and alkenes are similar in all of the following properties except
reactivity
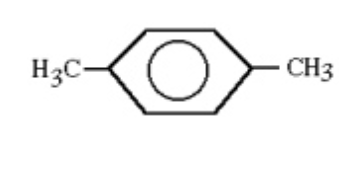
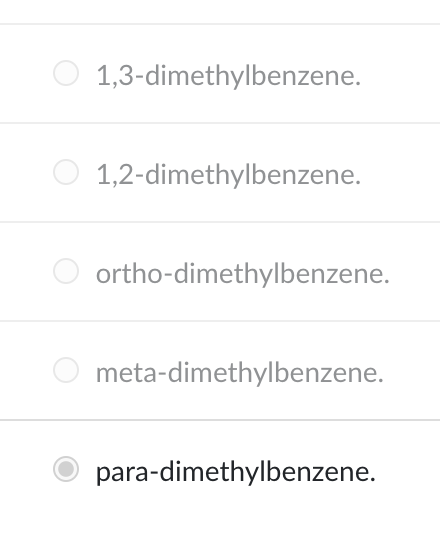
Using systematic names, the structure shown could be called
para-dimethylbenzene.
What is the IUPAC name of the molecule shown?
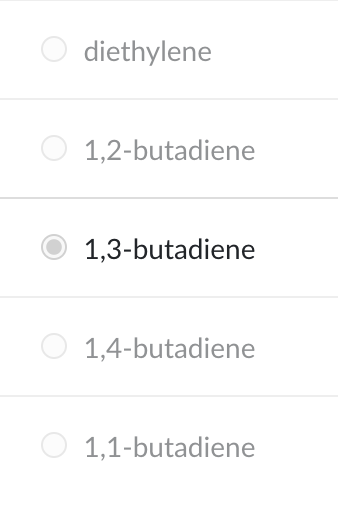
CH2=CH—CH=CH2
1,3-butadiene
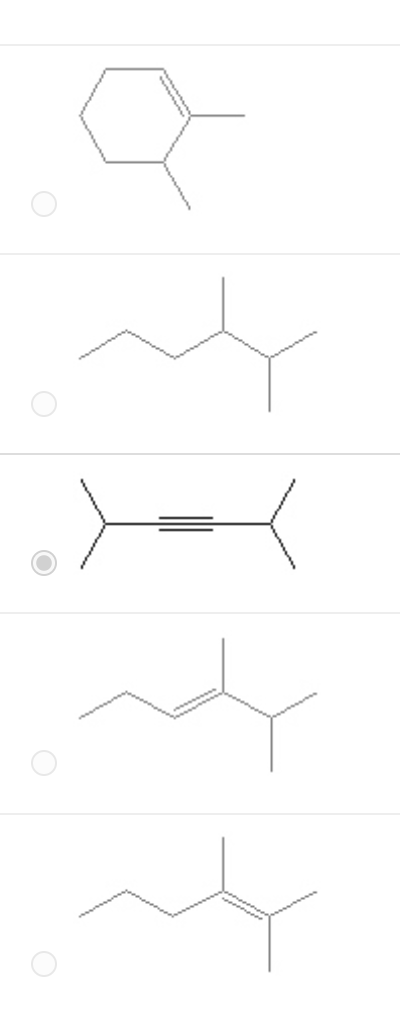
Which of the following is a correct line bond structure for 2,5- dimethyl-3-hexyne?
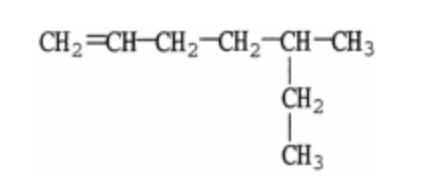
What is the IUPAC name of the molecule shown?
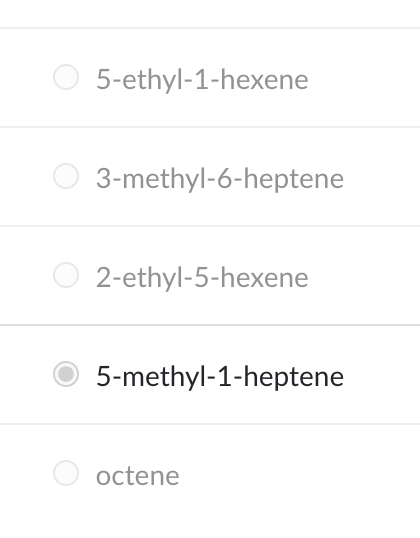
5-methyl-1-heptene
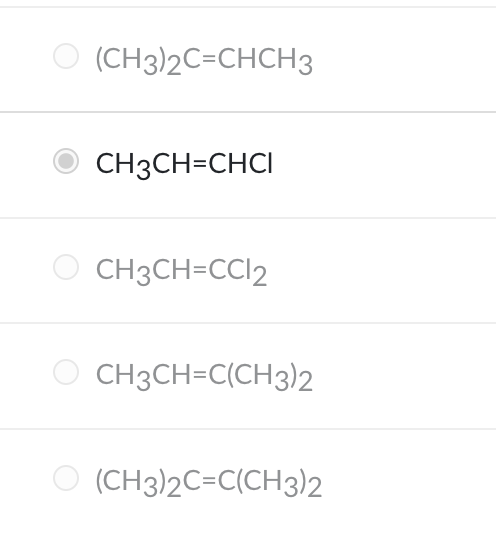
Which molecule can have cis-trans isomers?
CH3CH=CHCl
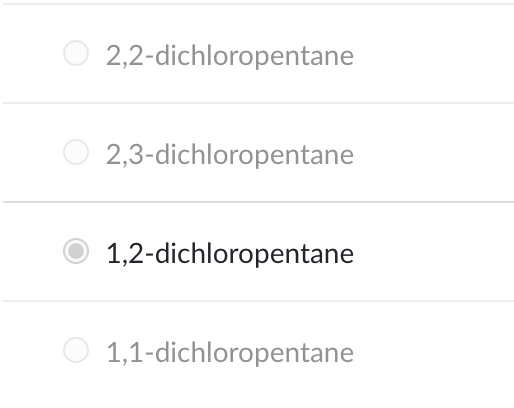
What is the name of the product when 1-pentene reacts with Cl2?
1,2-dichloropentane
In organic chemistry, the term unsaturated means a molecule
that contains one or more multiple bonds between carbon atoms.

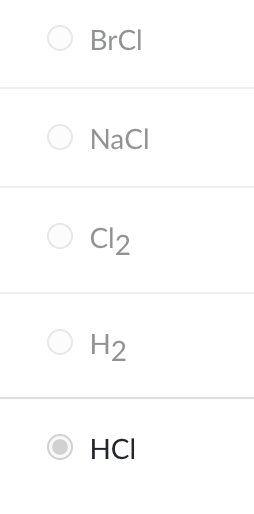
Which reactant should be used to convert propene to 2-chloropropane?
HCl
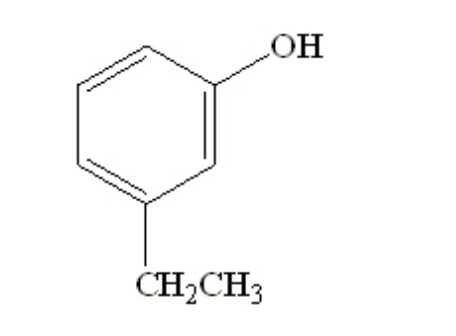
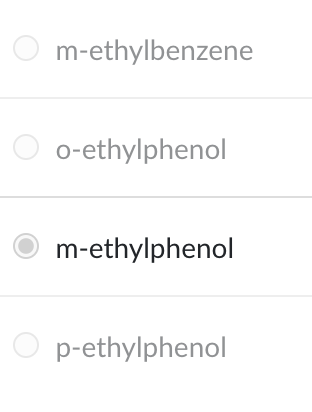
What is the name of the following compound?
m-ethylphenol
Chemical reactions involving double bonds are generally referred to as ________ reactions.
addition
A substitution reaction can best be described as a reaction in which
two reactants exchange atoms to give two new products.
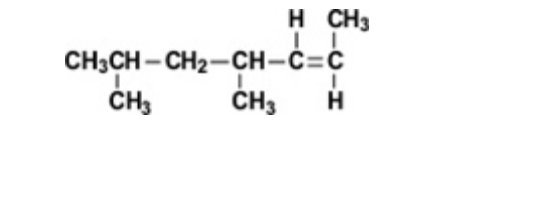
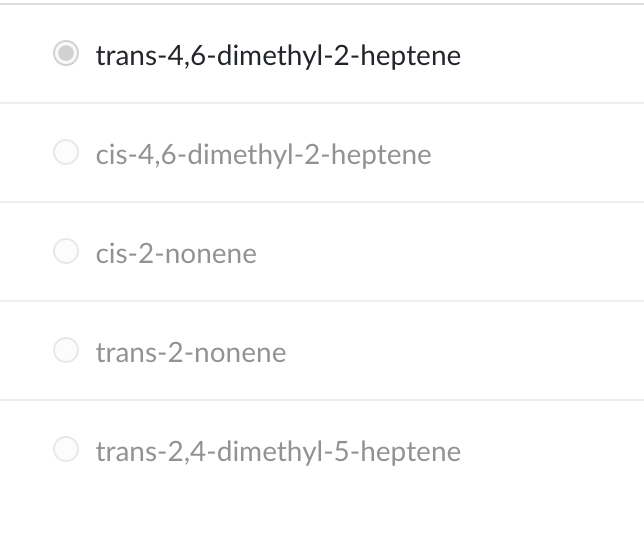
What is the IUPAC name of the compound shown?
trans-4,6-dimethyl-2-heptene