Alcohols
1/57
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
58 Terms
What is the alcohol functional group also called?
a hydroxyl group
What is the general formula of an alcohol?
CnH2n+1OH
What is the suffix for alcohols?
-ol
What is the alcohol functional group?
-OH
If an alcohol contains two hydroxyl groups, what do we call it?
a diol
Which functional groups have naming priority over the alcohol functional group?
aldehydes, ketones and carboxylic acids
What prefix is used to show the alcohol group in a molecule containing an alcohol and a higher priority functional group?
"hydroxy"
What are the three categories of alcohols?
primary, secondary, tertiary
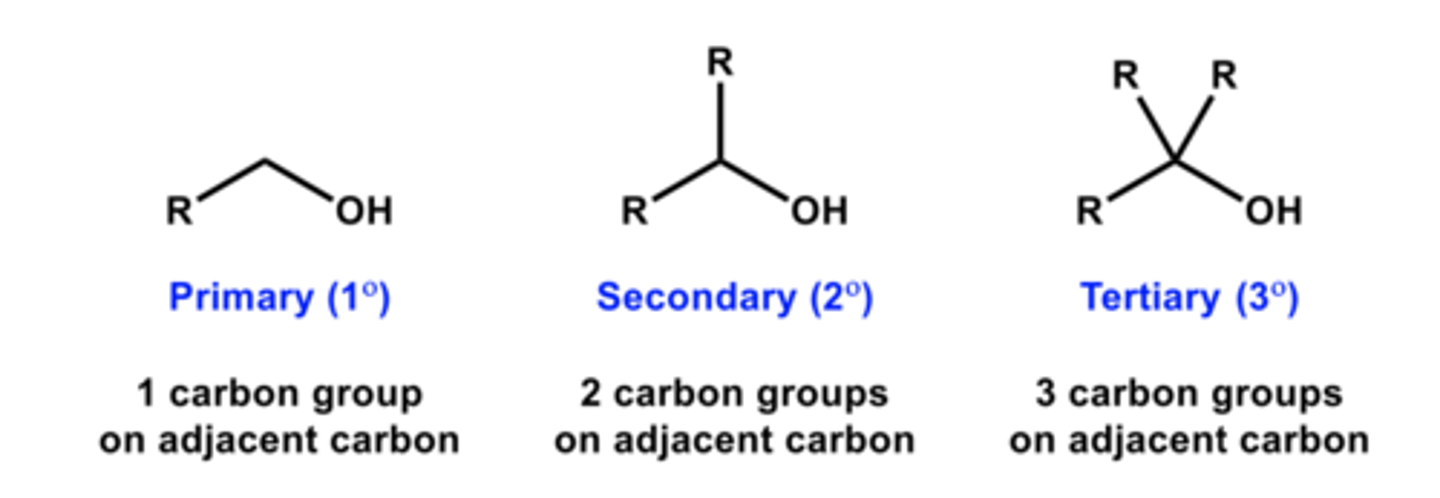
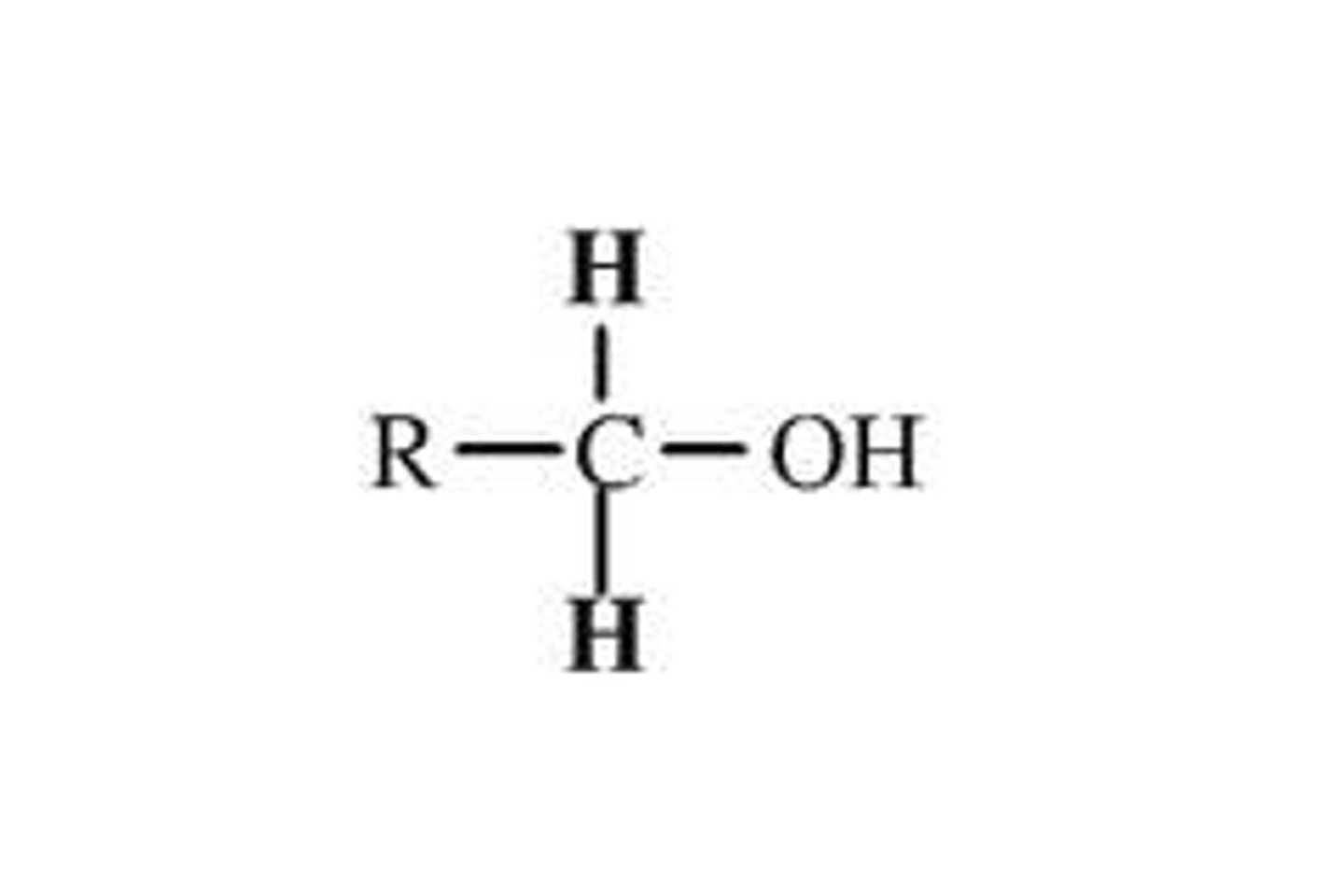
What are primary alcohols?
where the OH is attached to a carbon only attached to one other carbon
Give an example of a primary alcohol
ethanol
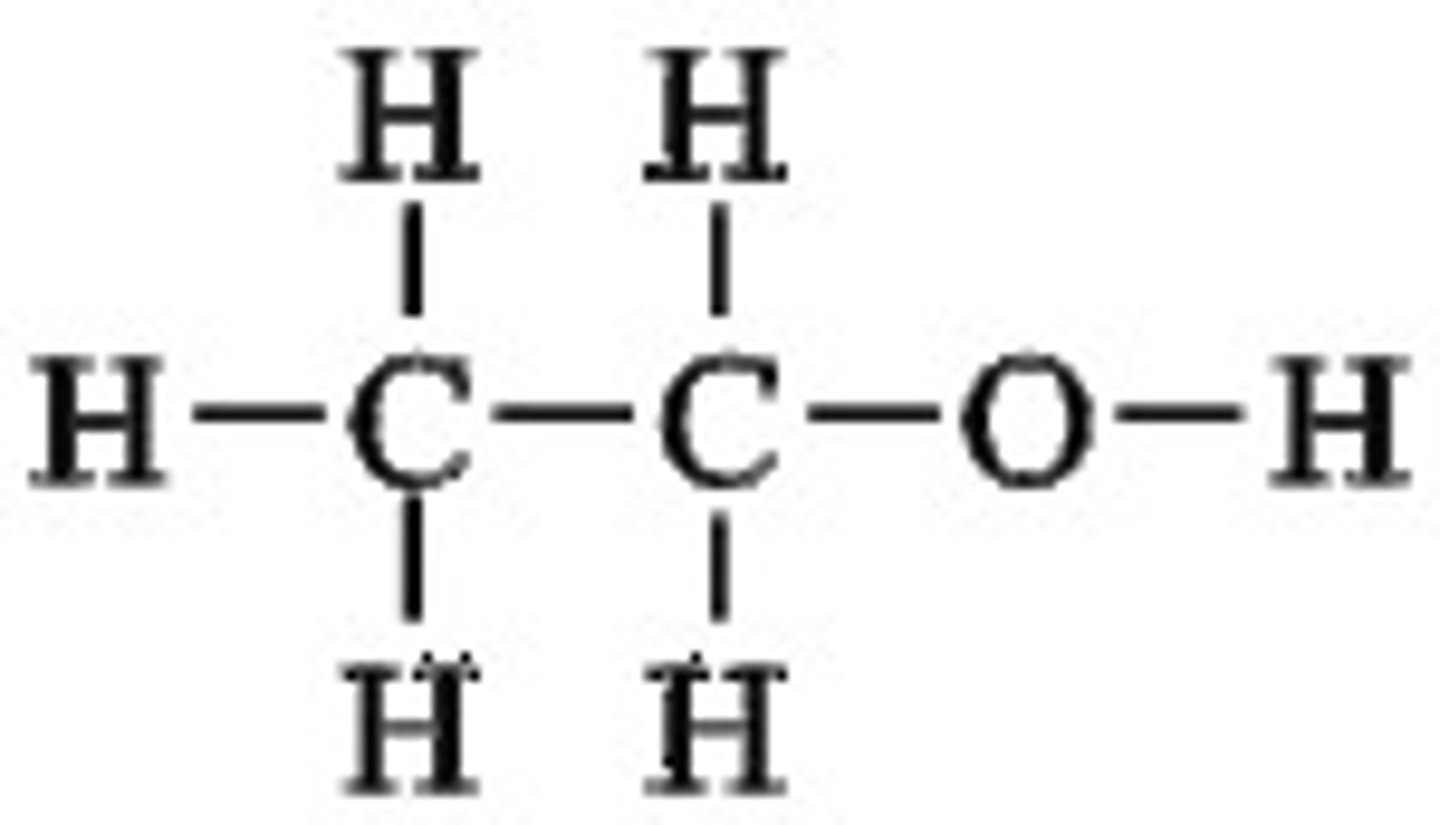
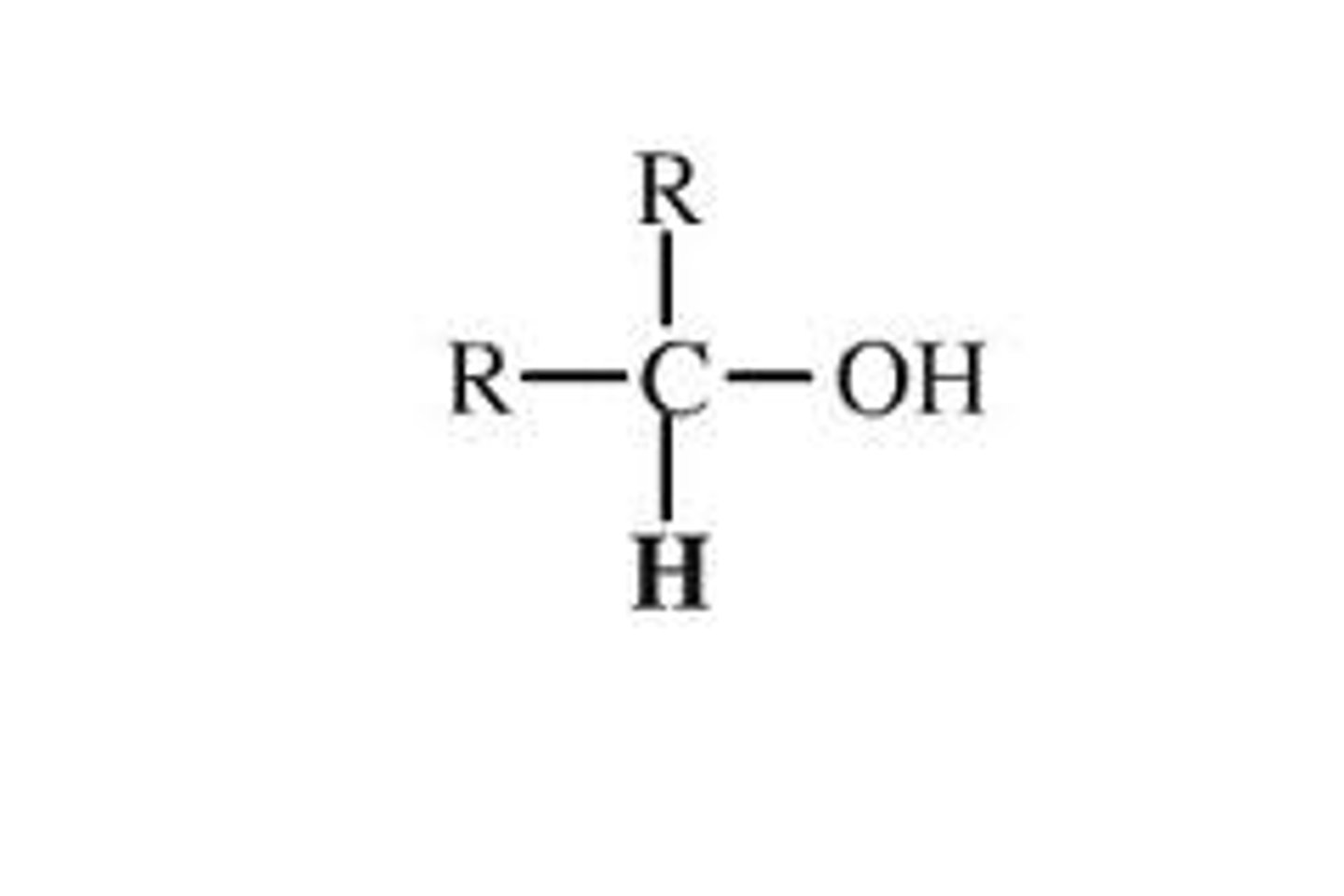
What are secondary alcohols?
where the OH is attached to a carbon attached to two other carbons
Give an example of a secondary alcohol
Propan-2-ol
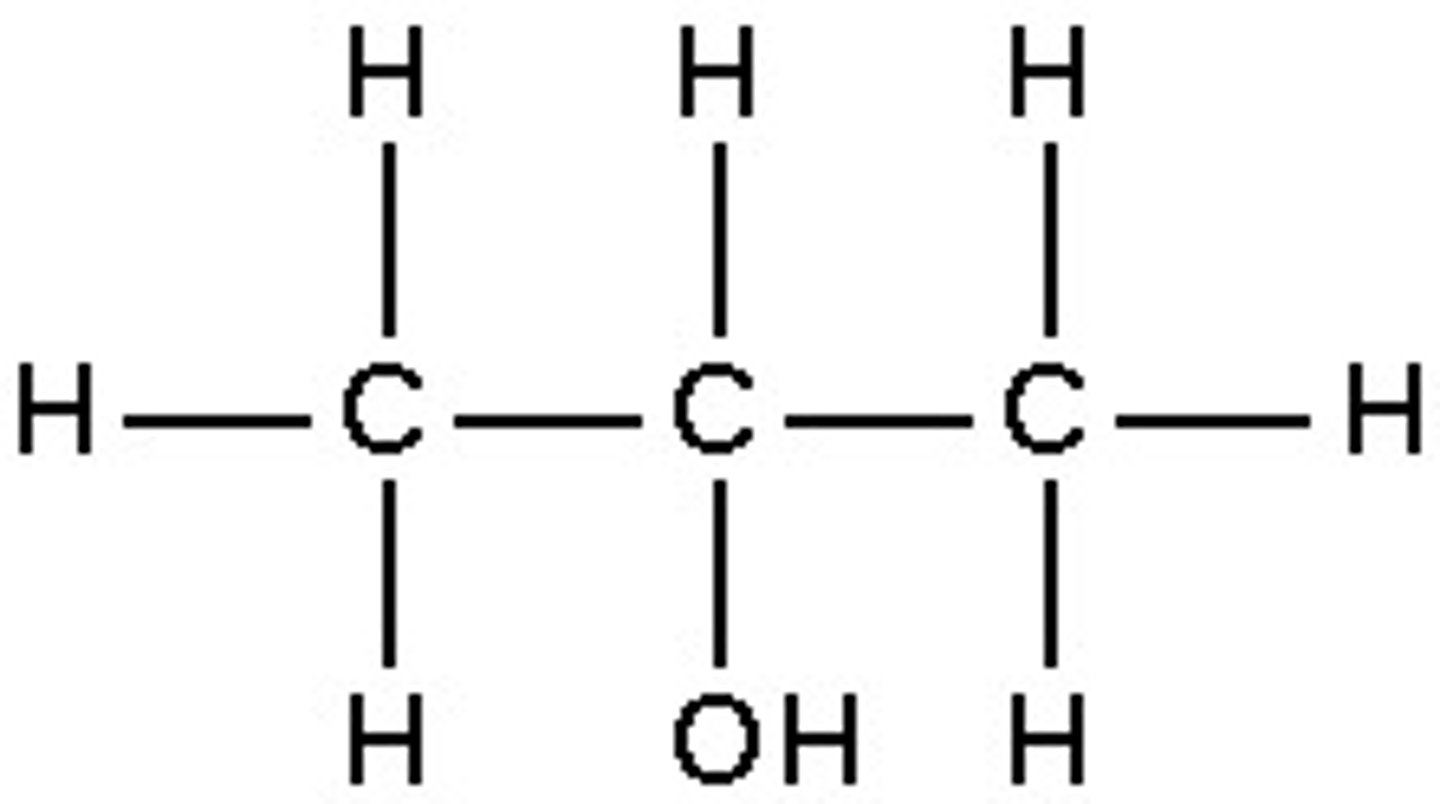
What is a tertiary alcohol?
where the OH is attached to a carbon attached to three other carbons
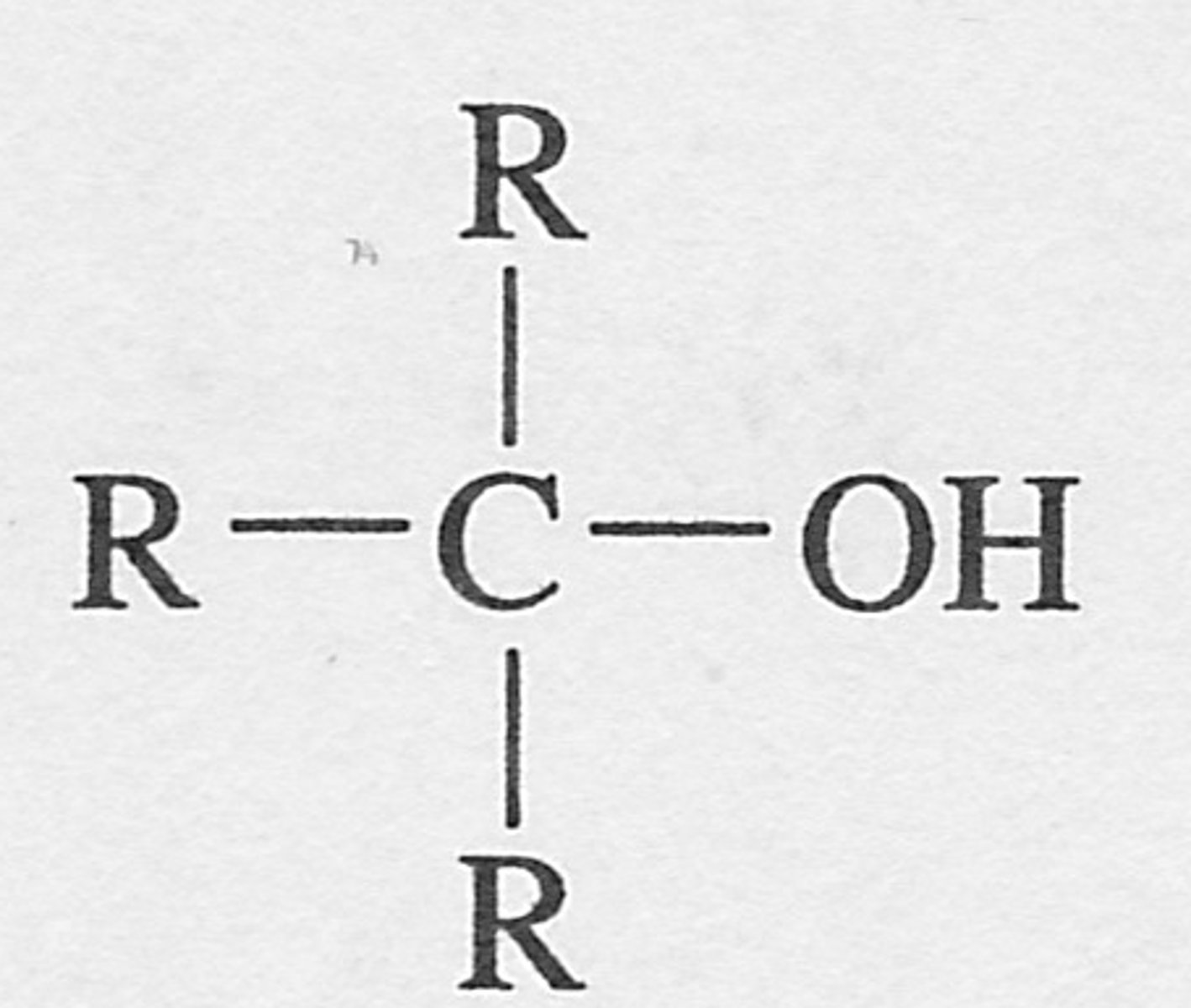
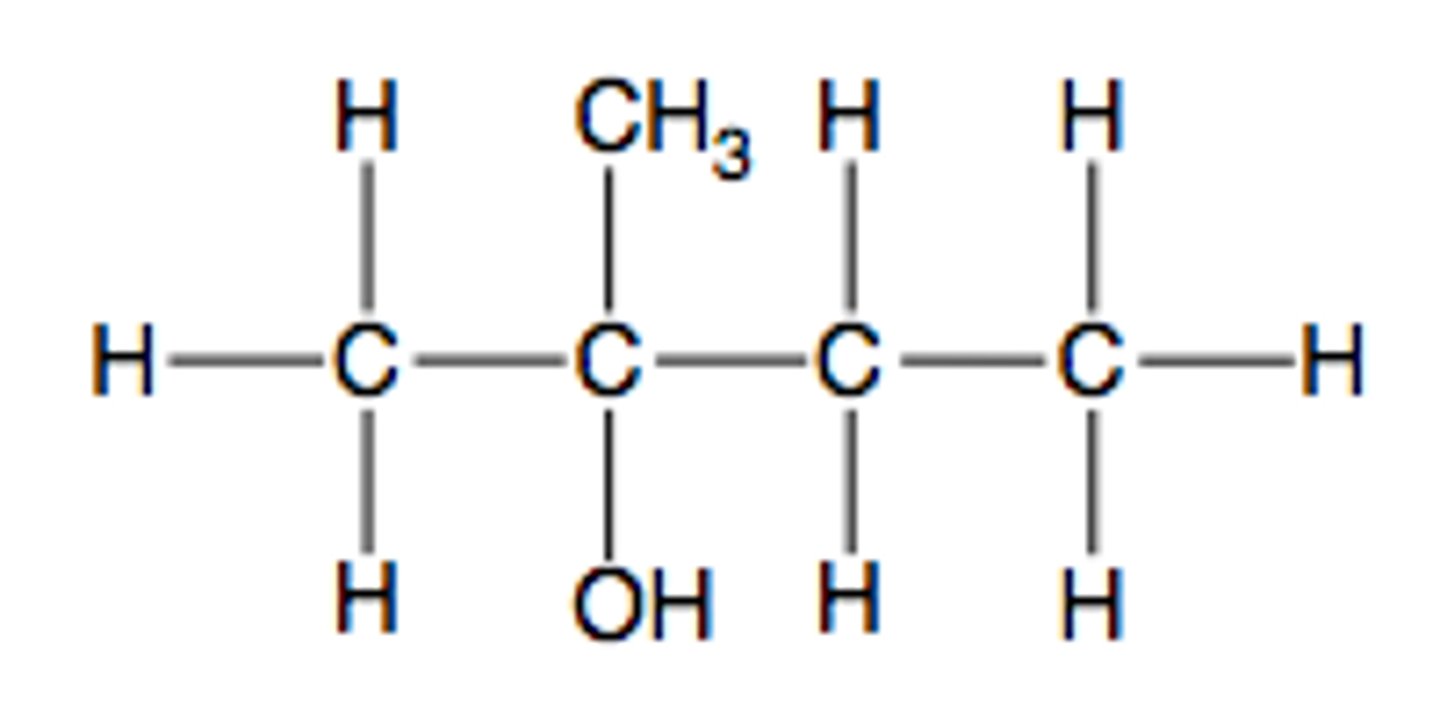
Give an example of a tertiary alcohol
2-methylbutan-2-ol
Why are alcohols less volatile (readily able to turn into gas) than alkanes?
Alcohols have higher boiling points
Why do alcohols have a higher boiling point than an alkane with the same number of carbon atoms?
Alkanes only have vdw forces (as they are non polar) whereas alcohols can form both vdw forces and hydrogen bonds
Why are alcohols highly soluble in water?
Because the alcohol functional group can form hydrogen bonds with water molecules
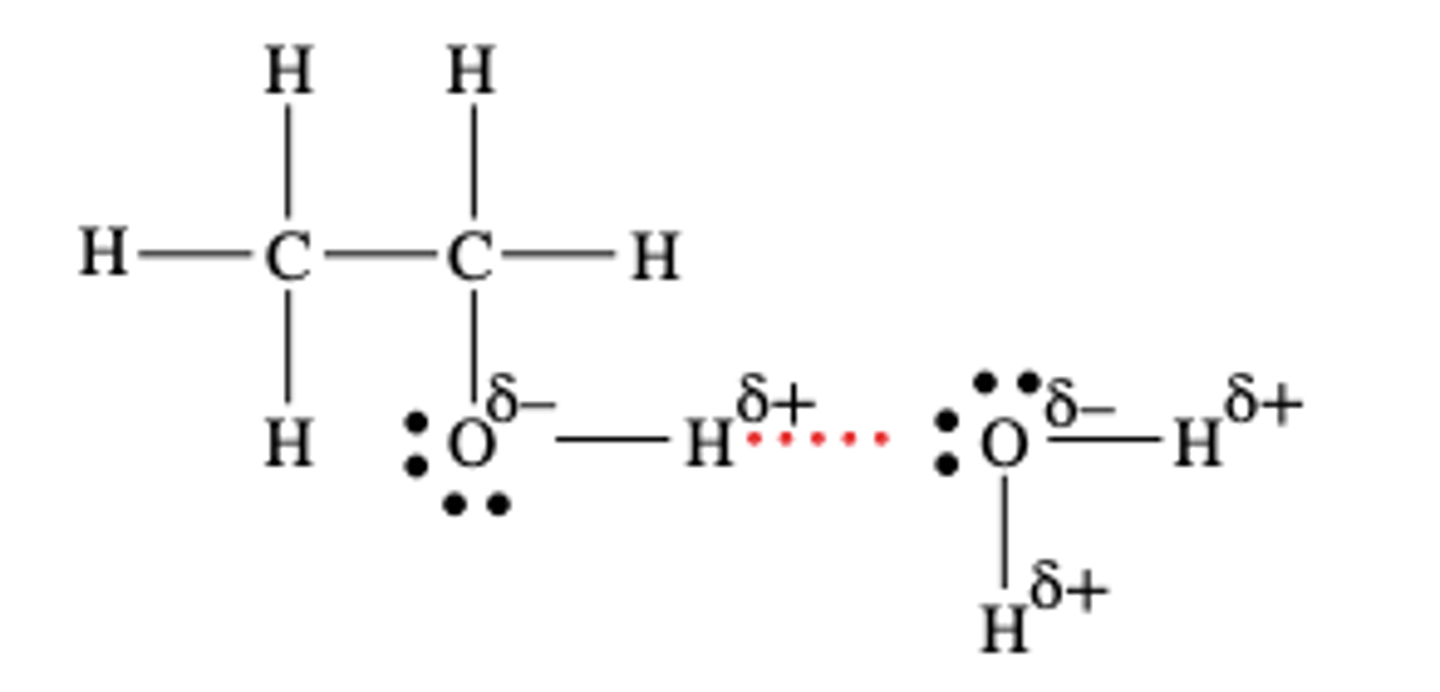
Why is it that as we increase the length of the carbon chain, alcohols become less soluble in water?
Because the non polar carbon chain can't form hydrogen bonds
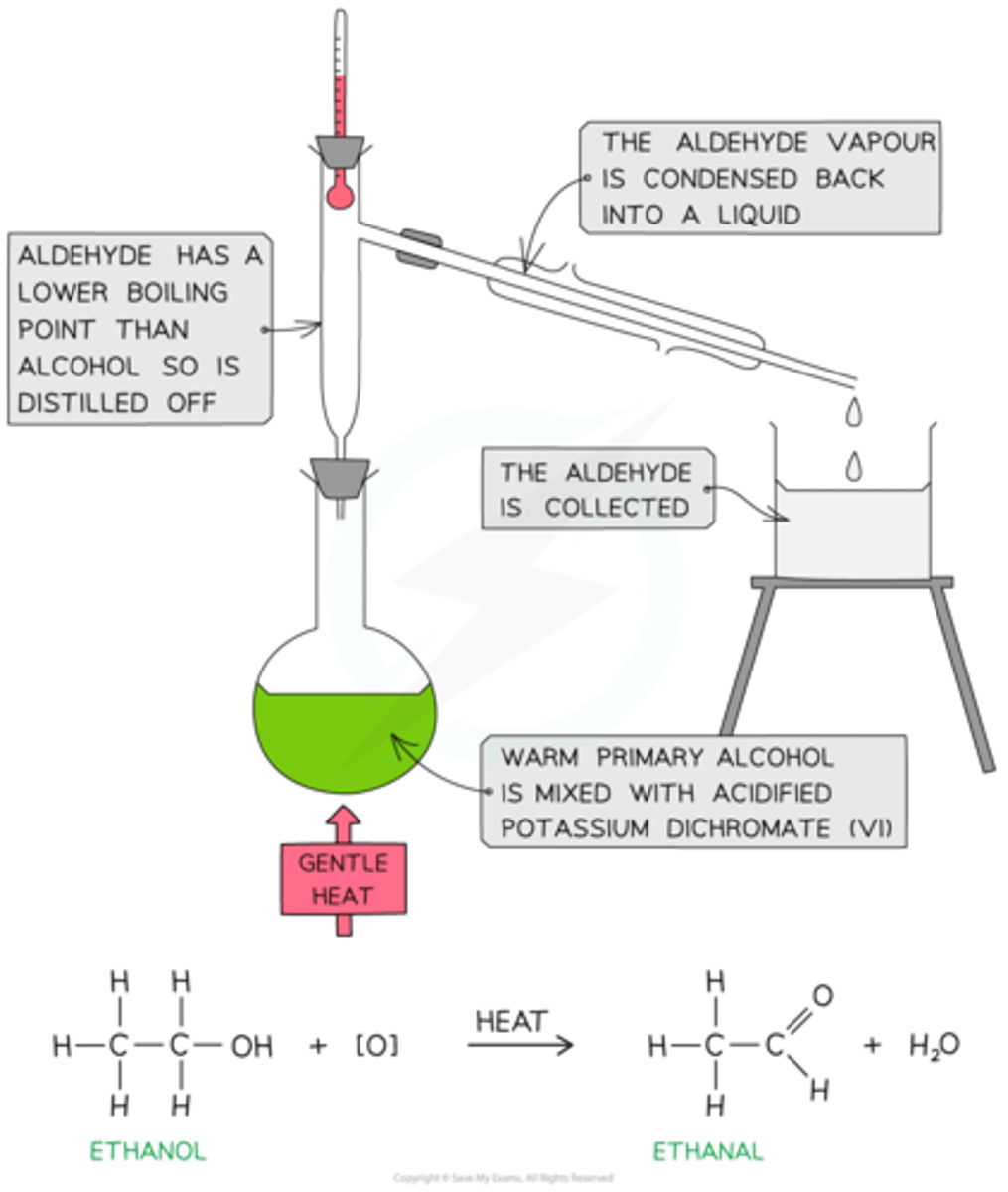
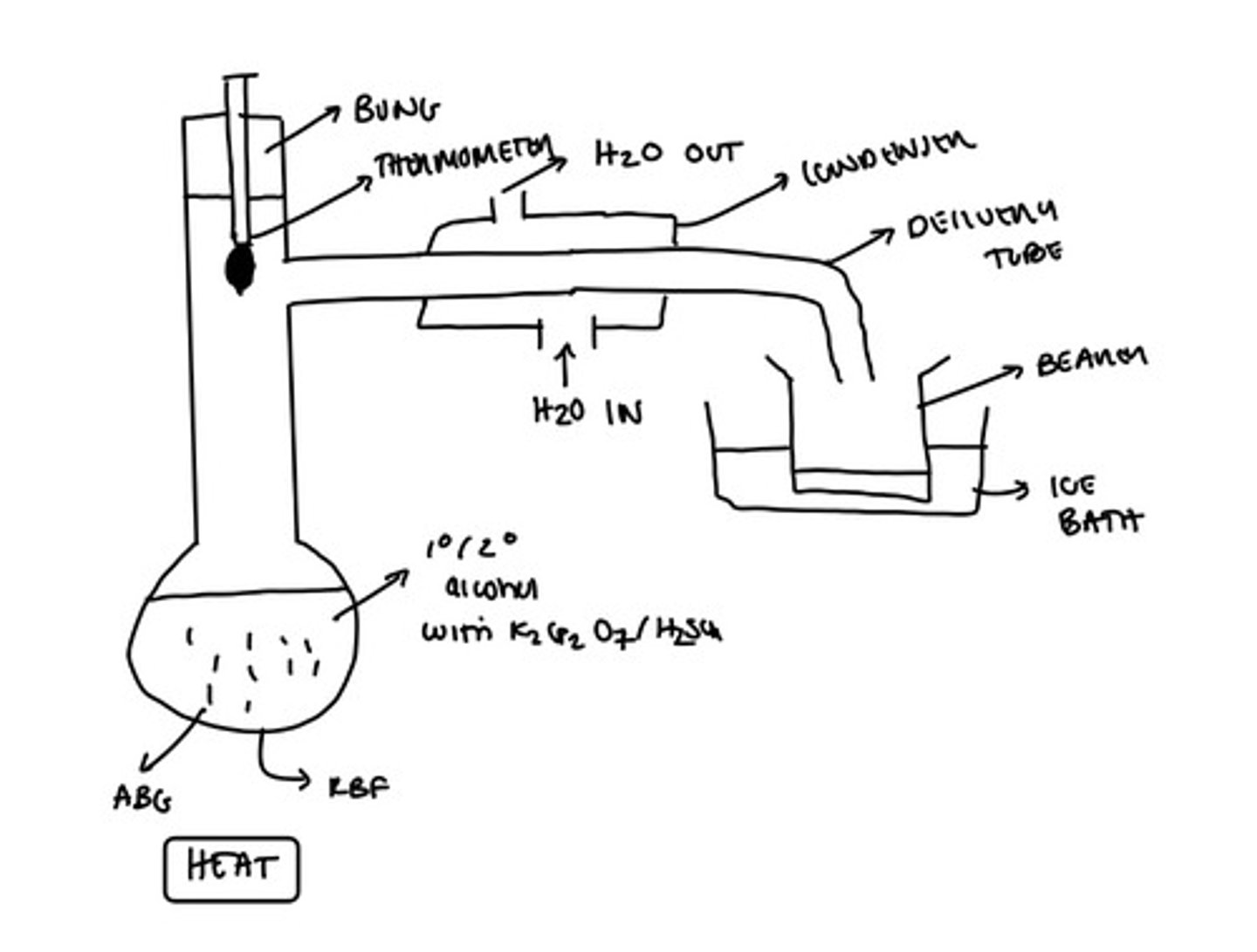
What is the product of oxidising a primary alcohol?
an aldehyde (and water)
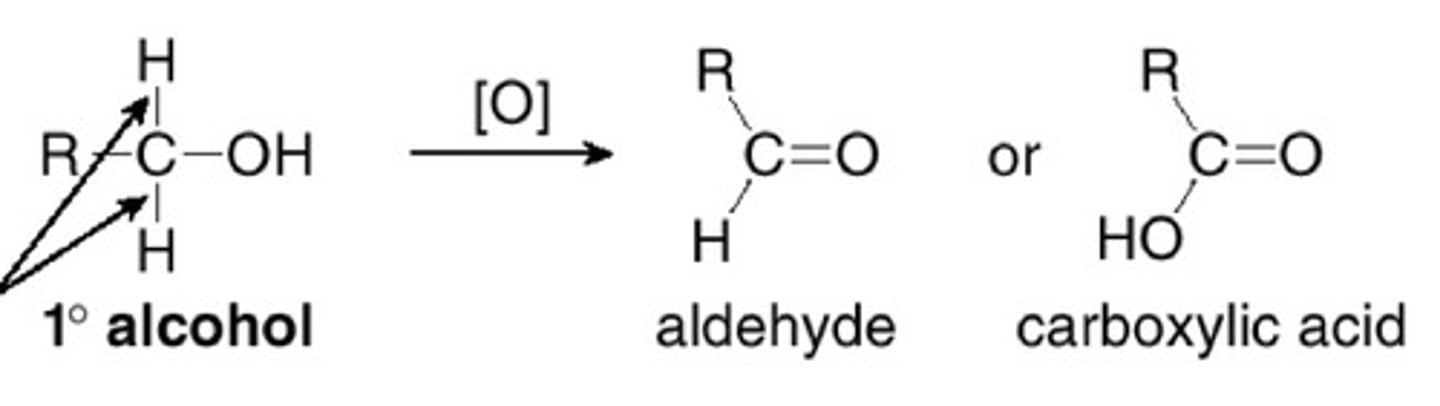
What is oxidation carried out with?
an oxidising agent
Give an example of a common oxidising agent
Potassium dichromate with dilute sulfuric acid (a.k.a acidified potassium dichromate)
What is the formula of acidified potassium dichromate?
K₂Cr₂O₇/H⁺
What do scientists often use to represent an oxidising agent?
[O] - Shows one molecule of oxidising agent is taking part in the reaction
How do we remove the aldehyde formed from oxidation of a primary alcohol?
Fractional distillation
What can aldehydes be oxidised to?
carboxylic acids
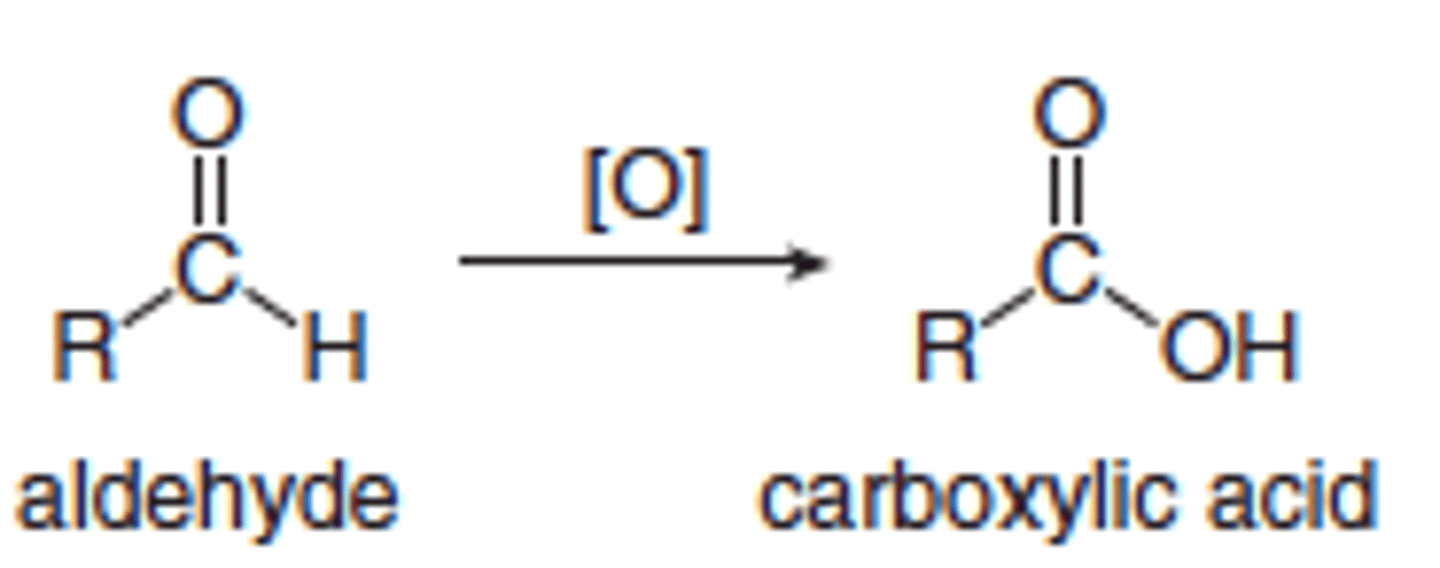
What is needed to oxidise an aldehyde into a carboxylic acid?
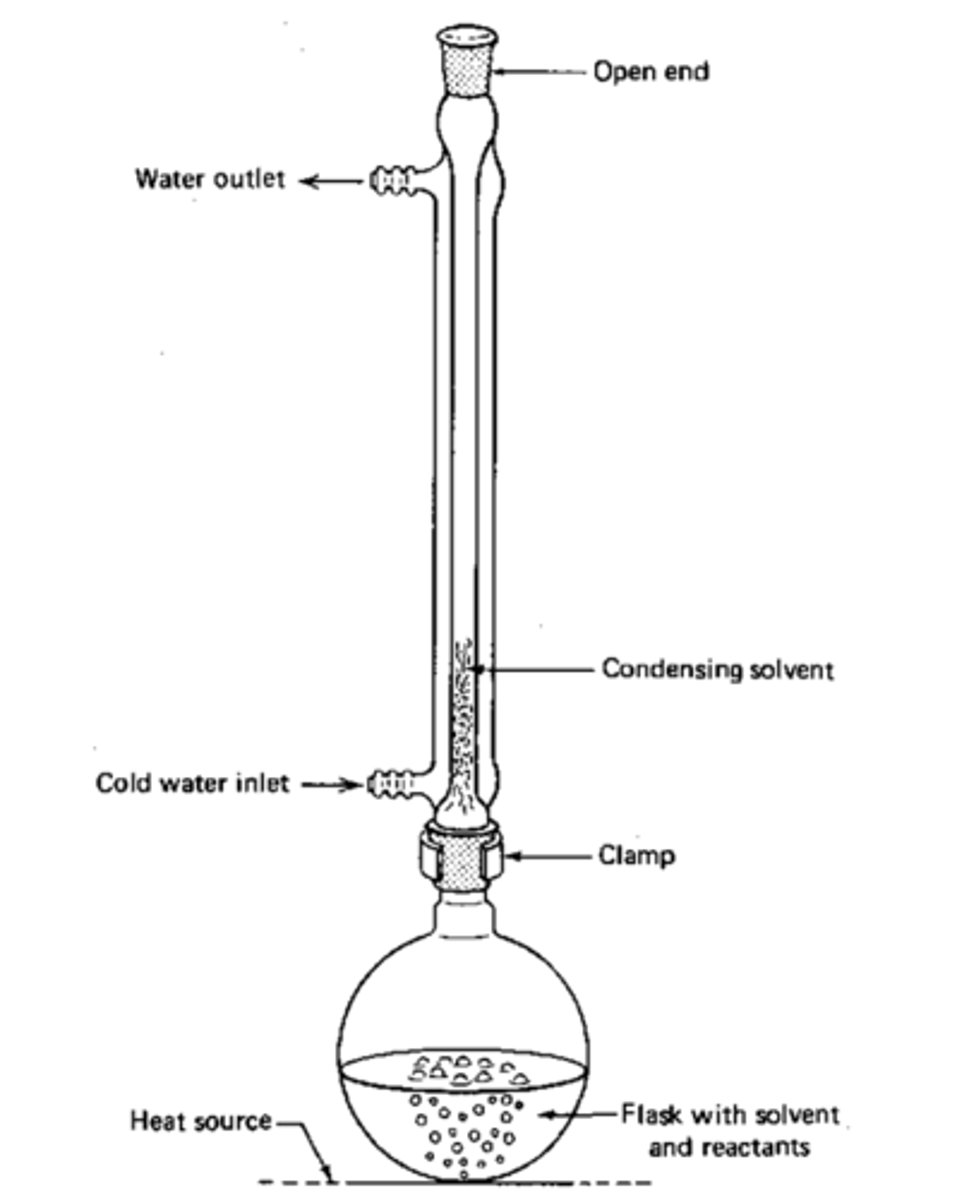
Excess of oxidising agent and reaction needs to be heated under reflux
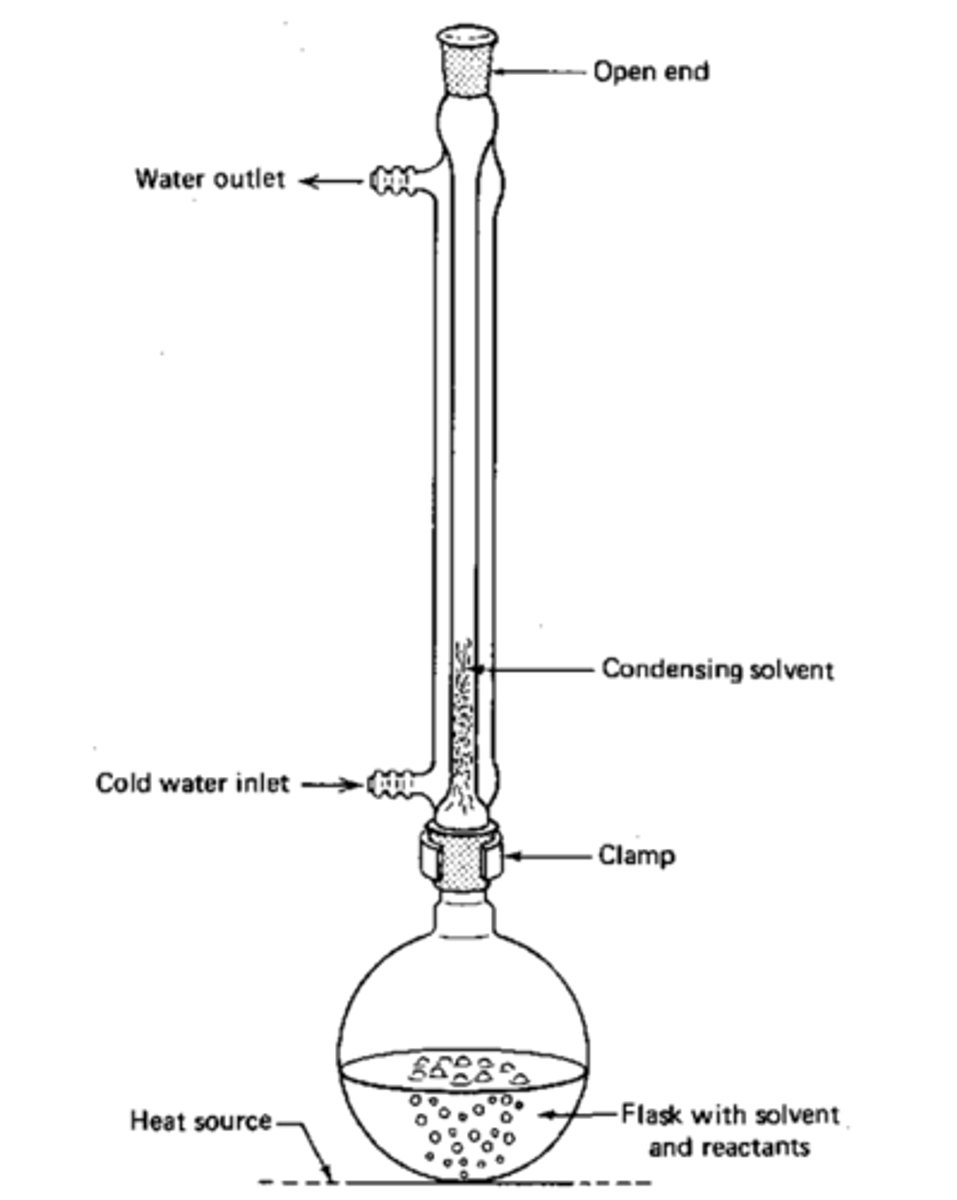
What occurs when we heat a reaction under reflux?
Any volatile products are condensed and return to the reaction mix
How can we separate carboxylic acids from our reaction mix?
Fractional distillation
What occurs during the oxidation of a primary alcohol if we use acidified potassium dichromate as the oxidising agent?
The dichromate (VI) ion is reduced to the chromium (III) ion and the solution goes from orange to green
What are the products of oxidising a secondary alcohol?
A ketone (and water)
Can ketones be oxidised any further?
No
What are the conditions for oxidising a secondary alcohol?
Heat the reactants under reflux - ensures as much of the ketone forms as possible
How do we separate ketones from our end reaction mixture?
using distillation
Why are ketones volatile chemicals with relatively low boiling points?
Ketones can't form hydrogen bonds
Can tertiary alcohols be oxidised?
no - acidified potassium dichromate remains orange
What does dehydration of alcohols produce?
alkene and water
What is the dehydration of alkenes an example of?
An elimination reaction
What occurs in an elimination reaction?
A small molecule is removed from a larger parent molecule
What is the small molecule removed from an alcohol in the dehydration of alcohols?
Water
What is the dehydration of alcohols facilitated by?
A heated acid catalyst
What does the complete combustion of alcohols produce?
carbon dioxide and water
What are two methods of ethanol production?
fermentation and hydration of ethene with steam
What is involved in hydration of ethene?
water is added to ethene in the presence of an acid catalyst.
How is ethene obtained?
cracking of crude oil
What acid catalyst is typically used in production of ethanol?
Phosphoric acid
What are the conditions used to create ethanol industrially?
- temperature of 300°C
- pressure of 60 atm
- presence of phosphoric acid catalyst
How is ethanol produced from fermentation?
Carbohydrates broken down into sugars then converted into ethanol by the actions of enzymes from yeast.
Write the equation for ethanol production from fermentation
C6H12O6(aq) ➔ 2C2H5OH(aq) + 2CO2(g)
What is the temperature used for fermentation?
35 degrees
Why is oxygen kept out of fermentation reactions?
If oxygen was present, yeast would respire aerobically, producing ethanoic acid instead of ethanol.
What is a biofuel?
A biofuel is a fuel derived from renewable biological sources.

How can ethanol produced by fermentation be described as a biofuel?
Glucose can come from sugar cane, which is renewable and biological.
What are the advantages of biofuels?
They are renewable and carbon-neutral
What are the disadvantages of biofuels?
Slower process made in batches and an aqueous solution of ethanol is produced
How can it be argued fermentation of glucose to produce ethanol is carbon neutral?
6 mol CO2 absorbed and 6 mol CO2 released
What are the arguments against fermentation of glucose being carbon neutral?
There are other carbon costs associated with the fuel, such as transport and processing.
Describe the Tollens' reagent test
Add Tollens' reagent to aldehyde/ketone and heat it in water bath. A silver mirror forms on the inside of the test tube for aldehydes.
Describe the Fehling's test
Add few drops of aldehyde/ketone to feeling's solution and put it in a water bath for a few minutes. An aldehyde goes brick red, and ketone no change.