Elements, compounds and mixtures (1.8 - 1.13) (copy)
1/14
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
15 Terms
What is an element?
Is a pure substance made up from only one type of atom
What is a compound?
A substance that contains two or more elements chemically joined together and cannot be separated by physical means.
What is a mixture?
Two or more substances that are not chemically combined and can be separated by physical means.
What is an atom?
The smallest part of an element that can exist
What is a molecule?
A particle that consists of two or more atoms chemically bonded together
melting and boiling points
a pure substance has a fixed melting and boiling point, but that a mixture may melt or boil over a range of temperatures.
e.g. water boiling point is 100, if it is impure it will be higher
Generally, impure substances have lower melting points and higher boiling points than the pure substance
Simple distillation
The solution is heated until the solution with the lowest boiling point starts to evaporate. The vapour is cooled and it condenses (turns back into a liquid) in the liebig condenser. This liquid can be collected, leaving the rest of the solution back in the flask.
What is simple distillation used for?
This method is used to separate a liquid from a solution
Fractional distillation
Fractional distillation works because the different liquids have different boiling points. When the mixture is heated:
vapours rise through a column which is hot at the bottom, and cold at the top
vapours condense when they reach a part of the column that is below the temperature of their boiling point
the liquid flows out of the column
What is fractional distillation used for?
Fractional distillation is used to separate different liquids from a mixture of liquids. It is useful for separating ethanol from a mixture of ethanol and water, and for separating different fractions from crude oil.
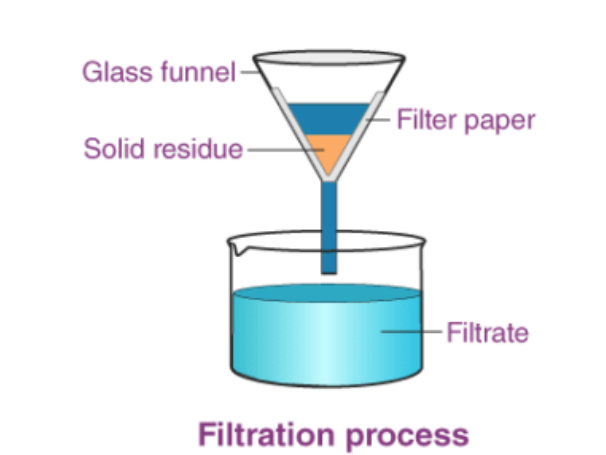
Filtration
to separate an insoluble solid from a liquid
The mixture is poured through filter paper, the liquid passes through the paper because of its small molecules, but the solid cannot because of its large molecules, leaving residue behind.
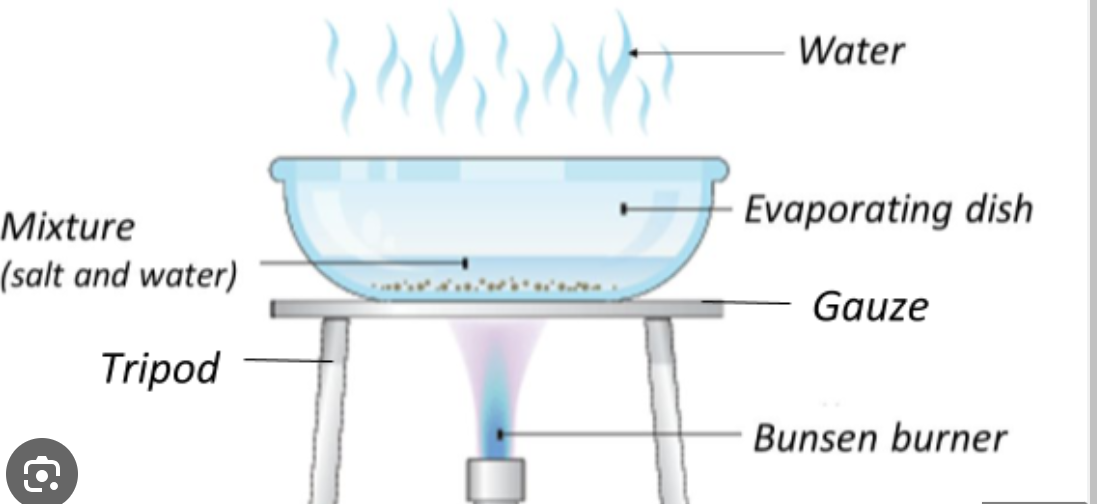
Crystalisation
to separate a soluble substance from a solvent
Heat up the solution (impure solid and solvent), in a evaporating dish, until most of the solvent is evaporated, then the solution can cool to form crystals, the crystals are then pressed between filter paper to dry.
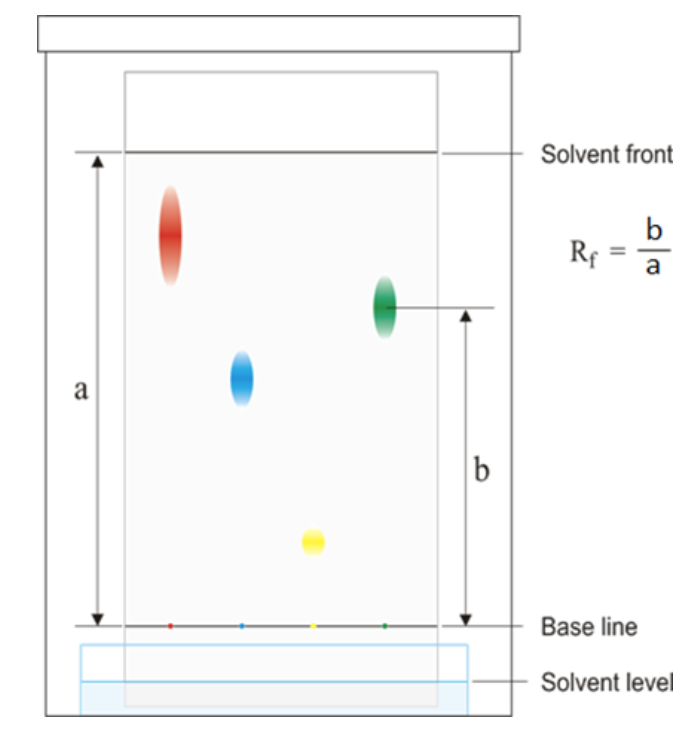
Chromatography
to separate different substances dissolved in a liquid
Chromatography method
1. Start by drawing a baseline on the chromatography paper using a pencil. The baseline is insoluble, so it won’t move with the solvent.
2. Place a small spot of the sample mixture on the baseline.
3. Pour the solvent into a beaker. Place a glass rod over the beaker, and tape or clip the paper to it, ensuring that the paper’s base just touches the solvent without being submerged.
4. Allow the solvent to slowly travel upwards through the paper, taking with it a few soluble pigments from the sample mixture. This will create different spots along the paper.
5. Remove the paper from the beaker before the solvent reaches the top. .
Rf value
Always has to be between 0-1