Construction of Neural Circuits Flashcards
1/90
Earn XP
Description and Tags
Flashcards on Neural Circuit Construction
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
91 Terms
Neurons are elaborated examples of…
Polarized epithelial cells
Epithelial cells are…
Found in most tissues
Assemble into sheets that absorb molecules from the environment in one domain and secrete proteins and other cells products in another
The first step in neuronal development is distinguishing the apical domain (axon) from the basal domain (dendrites)
Neuronal Polarization
The process by which neurons develop distinct apical (axon) and basal (dendrite) domains, similar to polarized epithelial cells.
Neuronal Processes: Once the neuroblast has entered a post mitotic state the outgrowth of neuronal processes begins
Neurites
Microtubule and actin cytoskeleton and other proteins are redistributes (Par 3)
Single process
Axon (apical domain express Tau [red])
Remaining Processes become dendrites (basal domain)
Neurites
Small extensions that protrude from immature neurons, before they differentiate into axons or dendrites.
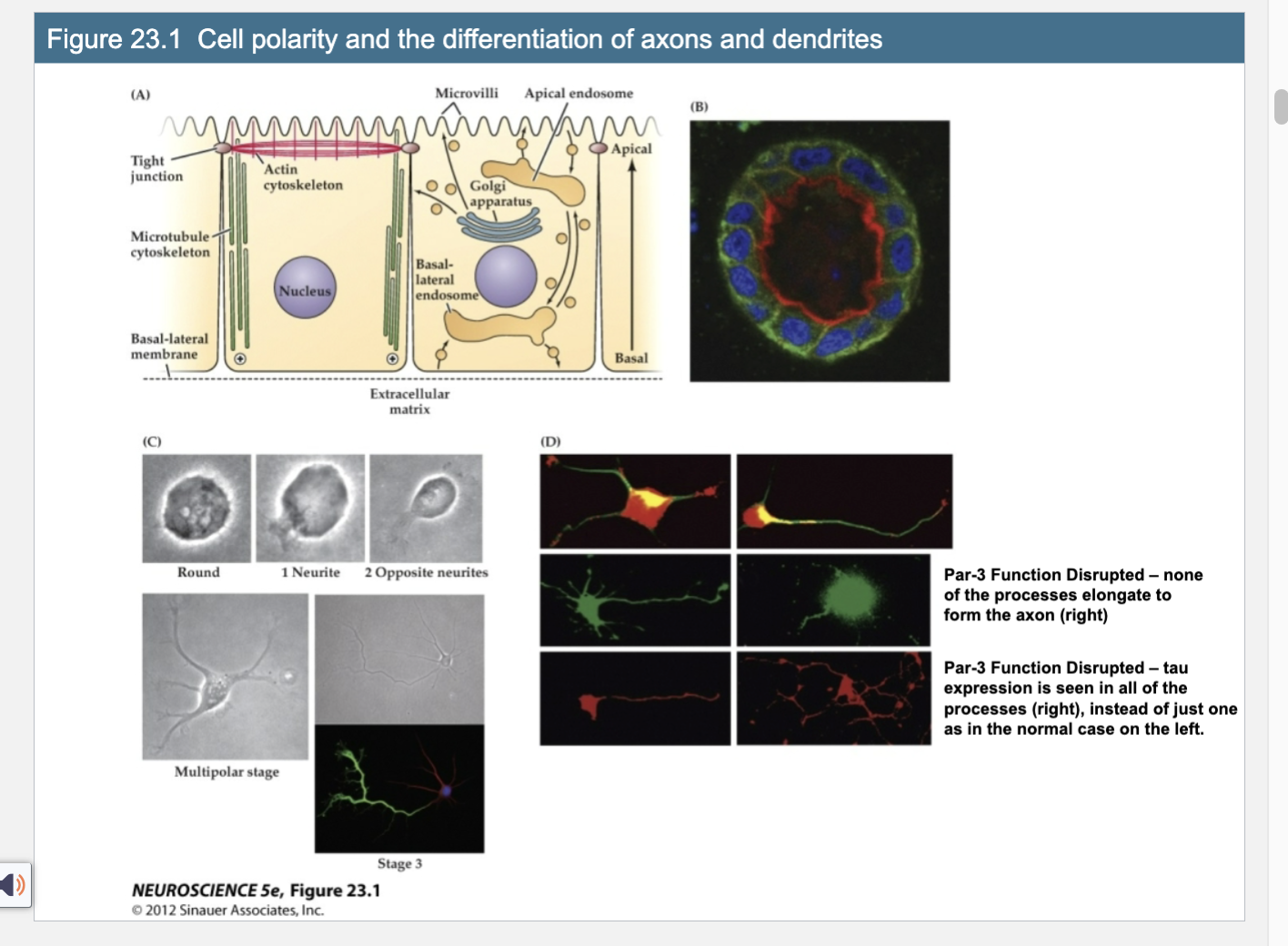
Figure 23.1- Cell Polarity and the differentatition of Axons and Dendrites
B. Red – actin apical domain; green – adhesion molecule (simple epithelium)
C. Neurite outgrowth; one neurite is the axon
D. Par- 3 – polarity scaffolding protein – found at the growing tips of neurites; normal middle left; par -3 distributed middle right, non of the processes elongate and tau is seen in all neurites, bottom right pic; tau is a microtubule associated protein found in single axon
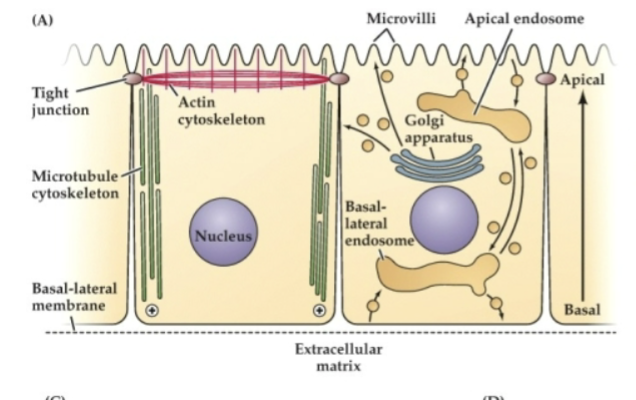
Explain Figure 23.1 Image A
(A) Polarized Epithelial Cell Structure (Diagram)
Cells like those in your gut have a clear top (apical) and bottom (basal) side.
Top (apical): has finger-like projections (villi), actin filaments, and is where the cell releases stuff (like secretions).
Bottom (basal): touches the outside environment (like tissue), has strong connections to other cells, and microtubules are arranged pointing down.
This structure helps the cell know which way is “up” and “down”—that’s cell polarity.
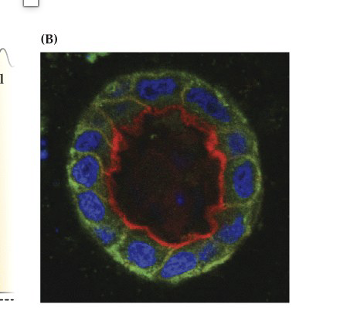
Explain Figure 23.1 Image B
(B) Real Microscopy Image of a Polarized Epithelial Tube
Red shows actin (concentrated at the top/apical side).
Green shows E-cadherin (an adhesion molecule found at the sides/bottom).
Confirms the cell is polarized—organized by top and bottom.
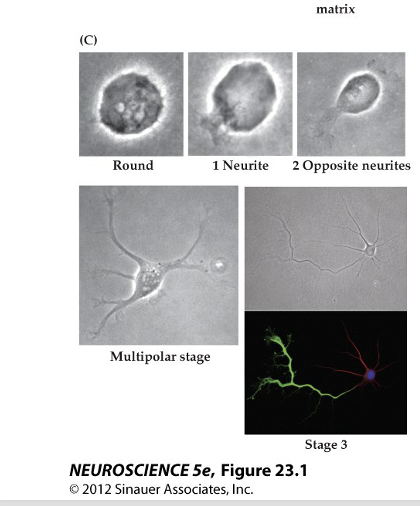
Explain Figure 23.1 Image C
(C) Developing Neuron – Neurite Formation
Shows a neuroblast (young neuron) growing extensions called neurites.
Starts round, then grows one neurite, then two opposite ones, and eventually multiple extensions.
One of these neurites will become the axon, while the others become dendrites—this is similar to how epithelial cells polarize.
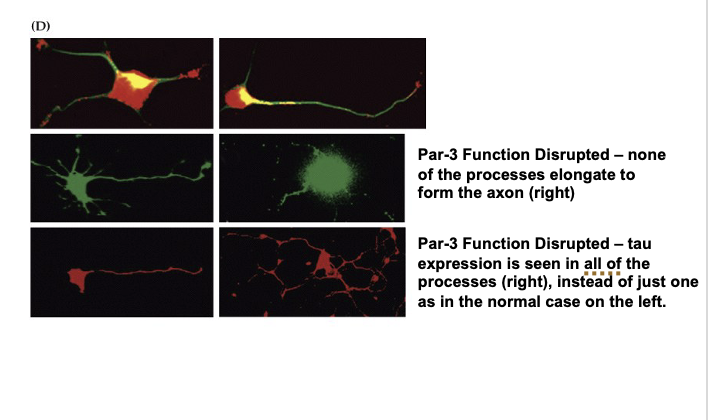
Explain Figure 23.1 Image D
(D) Role of Par-3 in Neuronal Polarity
Top row: Par-3 (red) is seen in all neurite tips early on—it's a key protein that helps determine which extension becomes the axon.
Middle left: In a normal cell, one process grows longer and becomes the axon (with Par-3 enriched there).
Middle right: When Par-3 is disrupted, no process grows into an axon.
Bottom left: Normally, Tau (a protein for axons) is only seen in one process (the axon).
Bottom right: If Par-3 is not working, Tau shows up in all processes, meaning the neuron can't decide which process should be the axon.
Key Takeaway:
Neurons become polarized just like epithelial cells, using proteins like Par-3 to decide which side becomes the axon. Without Par-3, neurons can’t organize themselves properly and fail to form a clear axon.
What does Figure 23.1 demonstrate about cell polarity and axon/dendrite differentiation in neurons?
It shows that neurons, like epithelial cells, develop polarity by distinguishing between the apical (axon) and basal (dendrite) domains. The polarity protein Par-3 is essential for this process: in normal neurons, Par-3 helps one neurite become the axon (marked by Tau). When Par-3 is disrupted, no axon forms, and Tau appears in all neurites, indicating a failure to establish polarity
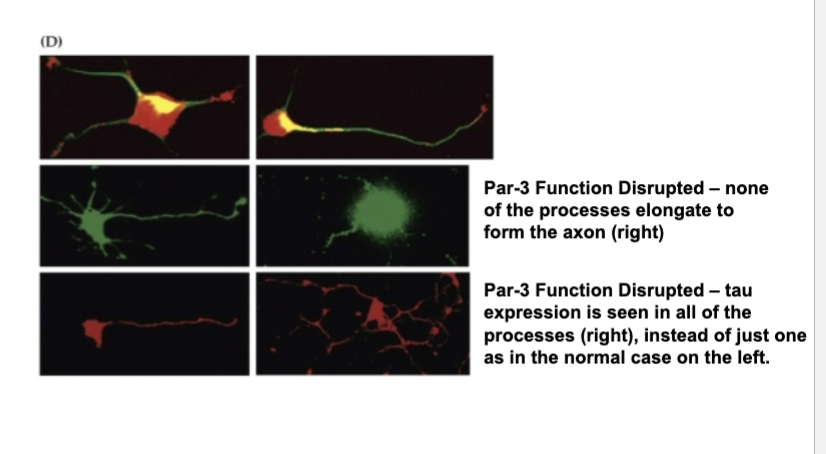
If Par3 function is disrupted then…
None of the processes elongate to form the axon (right)
If Par3 function is disrupted then (Tau)
Tau expression is seen in all of the processes (right), instead of just one as in the normal case on the left
Axon Growth Cone
A specialized structure at the tip of the growing neurite
3 Functions of Growth Cone
Explore the external environment
Determine the direction of growth
Guide the extension of the process in that direction
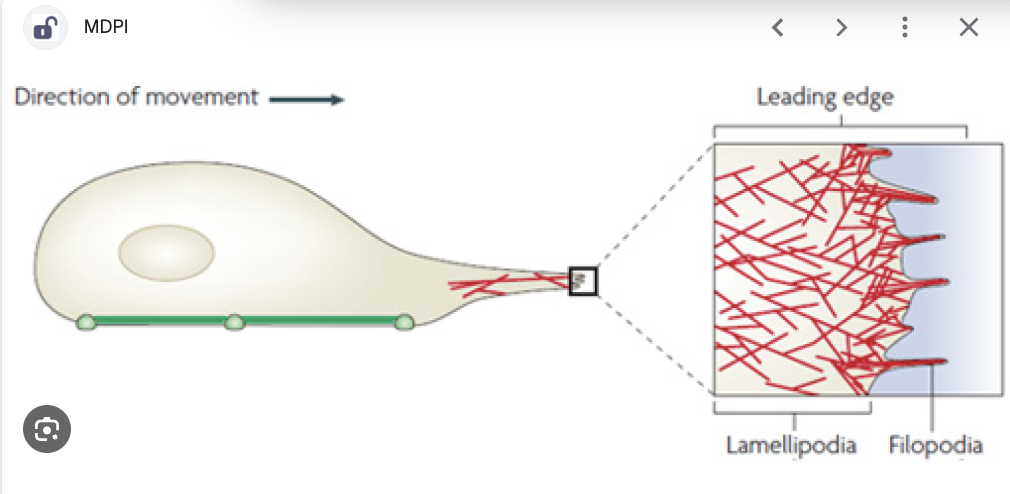
Lamellipodium
A sheet-like expansion of the growing axon, part of the growth cone structure.
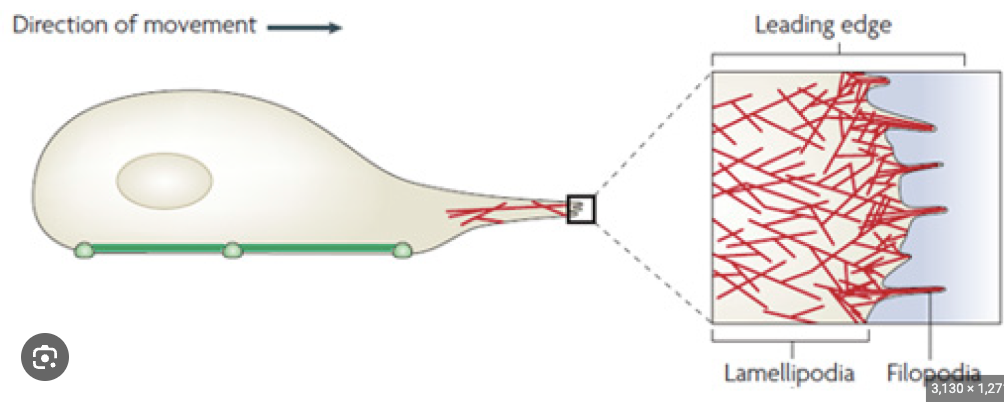
Filopodia
Fine processes that extend from the lamellipodium of a growth cone, used for exploring the environment.
Different Cytoskeletal Molecules than the axon shaft
Once growth cone reaches and recognizes an appropriate target…
It is transformed into a presynaptic ending for an axon
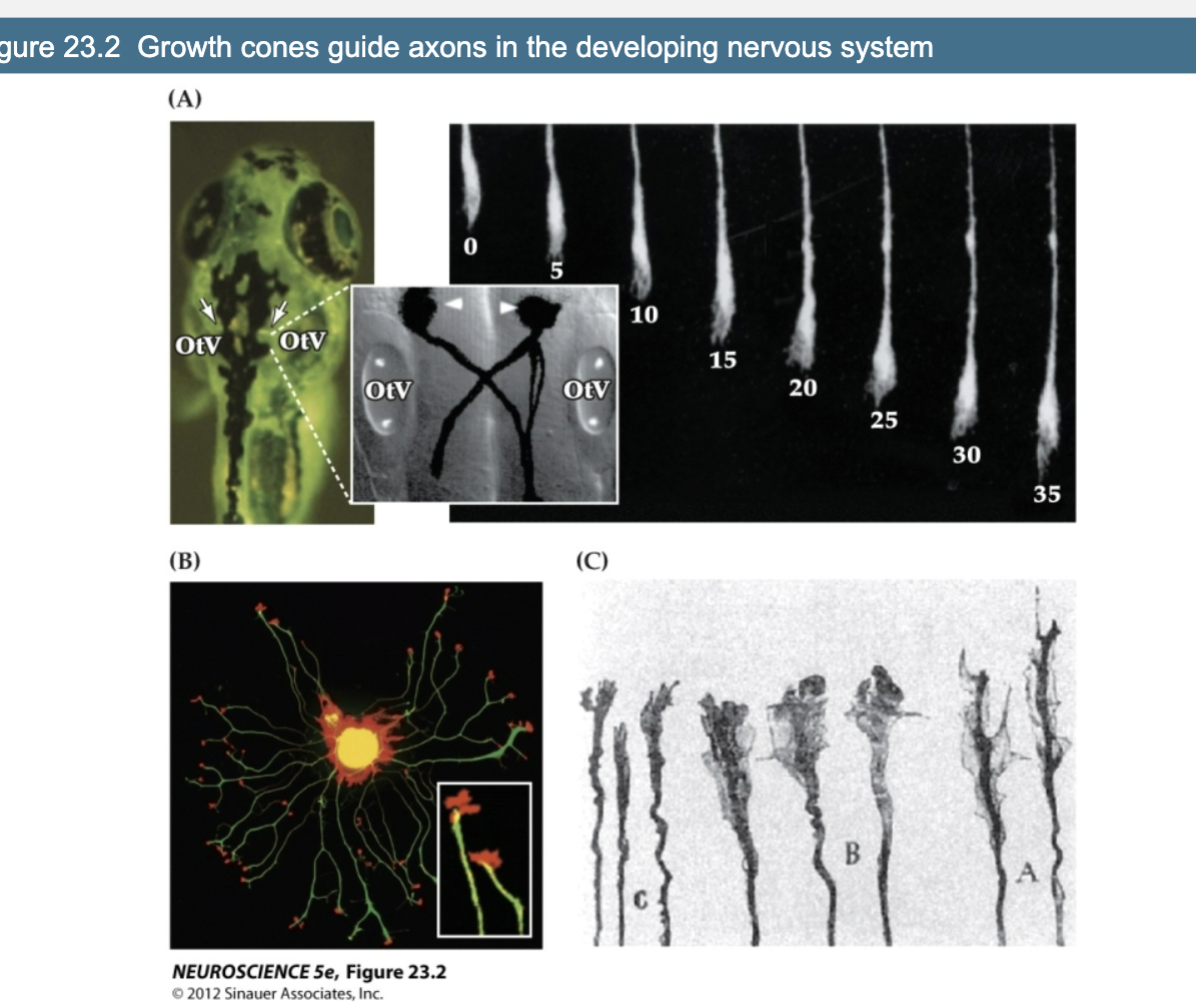
Explain Figure 23.2- Growth Cones Guide Axons in the Developing Nervous System
Growth cones are dynamic structures that guide axons through the developing nervous system. Their shape and structure change based on the task—exploring, making decisions, or continuing along a set path
(A) Zebrafish Embryo – Mauthner Neurons and Growth Cone Movement
The Mauthner neurons (arrows) are located near the ear vesicles (OtV) and grow axons that cross the midline and extend down the spinal cord.
The inset shows this crossing in more detail, emphasizing how axons can navigate the body.
On the right, we see a time-lapse of a single axon moving forward over 35 minutes, guided by a simple growth cone—this highlights how growth cones are active in navigating the environment.
(B) Isolated Dorsal Root Ganglion (DRG) Neuron in Culture
This neuron has extended many neurites, each ending in a growth cone.
The axon shaft contains microtubules (green), which give it structure and help with transport.
The growth cone at the tip contains actin (red), which allows it to change shape and move.
The inset zooms in on a single growth cone—showing its complex, branched structure used for sensing the environment.
(C) Growth Cone Morphology at Decision Points
Growth cones change shape depending on their location and the choices they need to make.
Near the origin (label C), growth cones are simple.
As they approach a decision point (like crossing the midline, labels B and A), the growth cones become larger and more complex, helping them make the correct directional choice.
Once the crossing is complete, they return to a simpler shape, resuming forward movement.
The Molecular Basis of Growth Cone Motility consists of…
Rapid, controlled rearrangement of the cytoskeleton (actin and myelin)
Actin Cytoskeleton (actin)
Regulates changes in lamellipodial and filopodial shape for directed growth of the axon.
Microtubule Cytoskeleton (tubulin)
Responsible for the elongation of the axon itself
4 Components and Functions of Microtubule Cytoskeleton (tubulin)
Elongation of axon itself
Microtubules run parallel to the axis of the axon
Structural Integrity
Transport of proteins fro the cell body to the axon terminal
Polymerization and depolymerization is regulated by…
Several Proteins
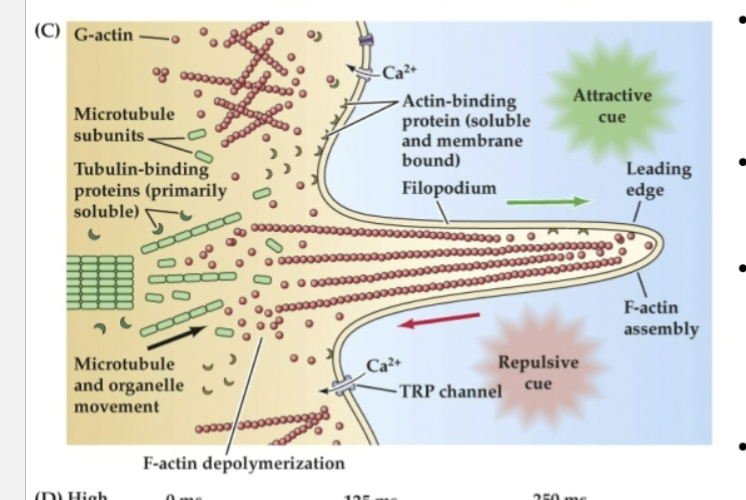
What does Figure 23.3 reveal about the structure and function of growth cones?
It shows that growth cones uses:
F-actin (red) in their outer regions for movement
Tyrosinated microtubules (green) in the lamella for structure
Acetylated microtubules (blue) in the axon shaft for stability.
Attractive cues promote actin assembly at the leading edge, while repulsive cues trigger disassembly.
These processes are regulated by intracellular calcium changes and cytoskeletal-binding proteins.
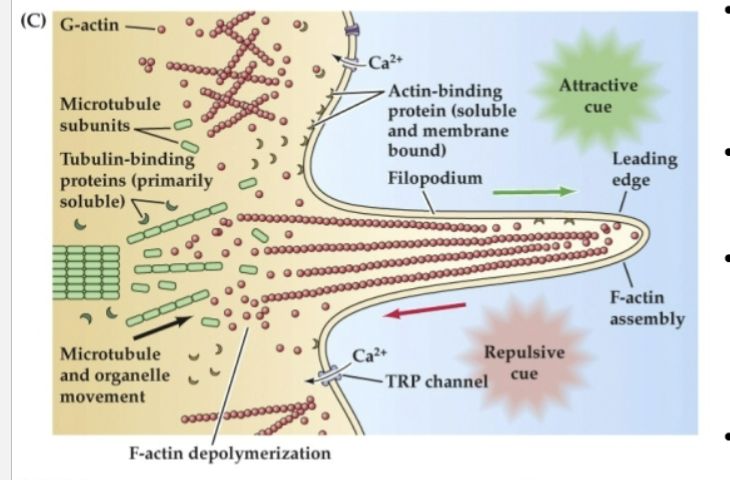
Globular actin (G-actin) can be incorporated into…
F-actin at the leading edge of a filopodium in response to attractive cues.
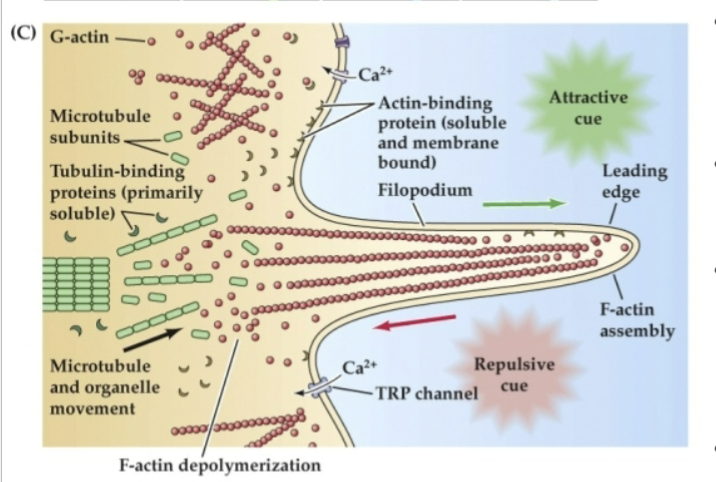
Repulsive cues support…
disassembly and retrograde flow of G-actin toward the lamellipodium
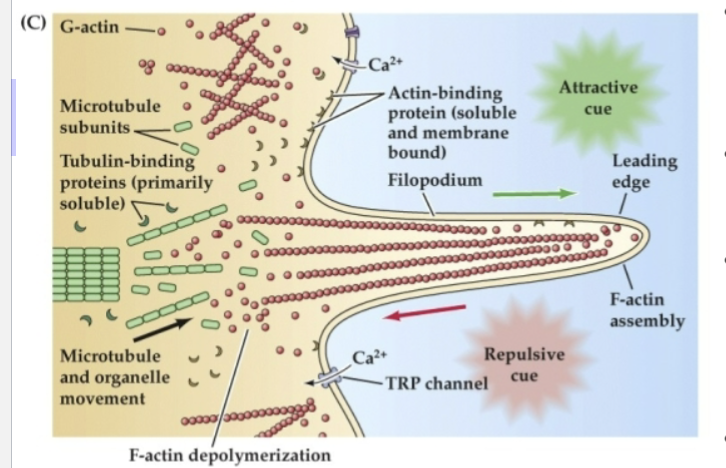
Organized microtubules make up the cytoskeletal core of the axon, while more broadly dispersed microtubule subunits are found…
at the transition between the axon shaft and the lamellipodium
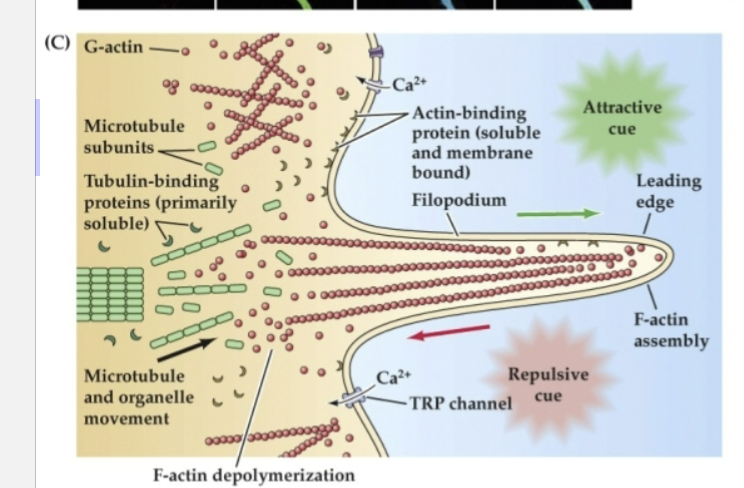
Actin- and tubulin-binding proteins regulate…
the assembly and disassembly of subunits to filaments or tubules
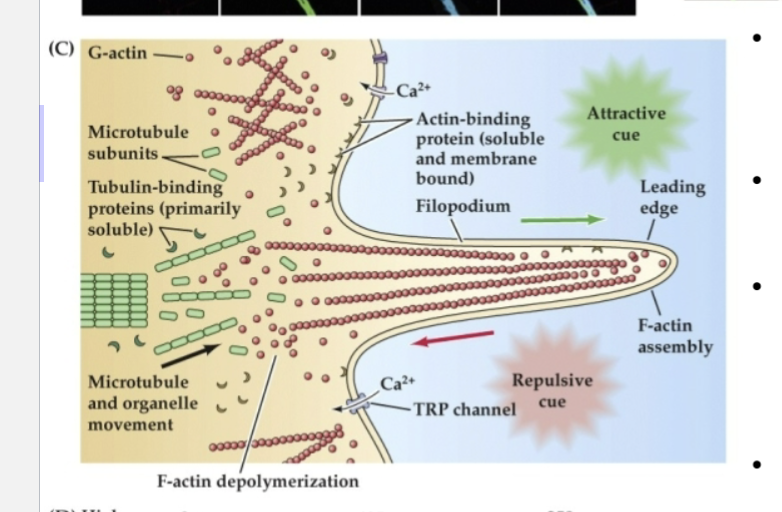
This process is influenced by changes in…
intracellular Ca2+ via voltage-regulated Ca2+ channels as well as transient receptor potential (TRP) channels
Signals for axon guidance- Specific cues cause the growth cone…
to move in a particular direction
Signals for axon guidance- …. What molecules respond to cues that cause the growth cone to move in specific directions?
receptors and transduction molecules
What can receptor and transduction molecules that respond to cues that cause the growth cone to move in specific directions cause?
an influx in Calcium (Ca2+) ions
Molecules known to influence axon growth and guidance can be…
grouped into families of ligands and their receptors
4 type of ligand rand receptors that constitute the major classes of axon guidance
Extracellular Matrix Molecules
CAMS
Cadherins
Ephrins
These ligand–receptor pairs can be either…
attractive or repulsive, depending on the identity of the molecules and the context in which they signal the growth cone.
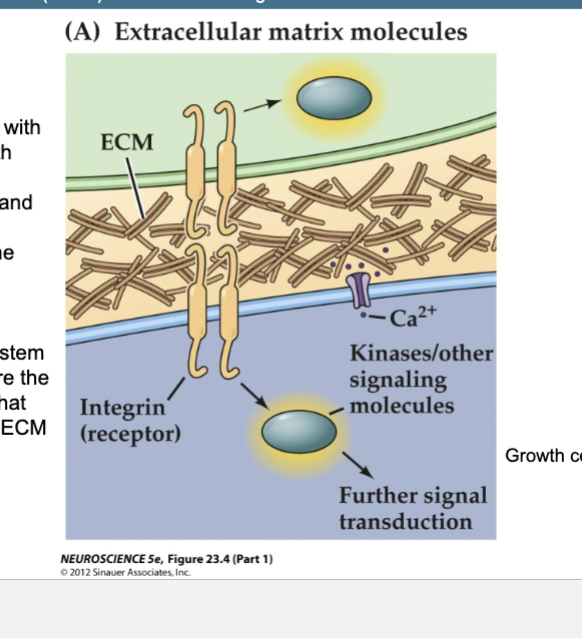
Figure 23.4- Several families of ligands and receptors constitute the major classes of axon
guidance molecules (Part 1) – Non-diffusible signals
Extracellular Matrix Molecules
1st to be associated with axon growth
Laminins, collagens, and fibronectin
Found in the ECM
Embryonic peripheral nervous system
Integrins are the receptors that bind these ECM molecules
Growth cone = purple
Integrins
Receptors that bind to ECM molecules like laminins, collagens, and fibronectin, important for axon growth and guidance.
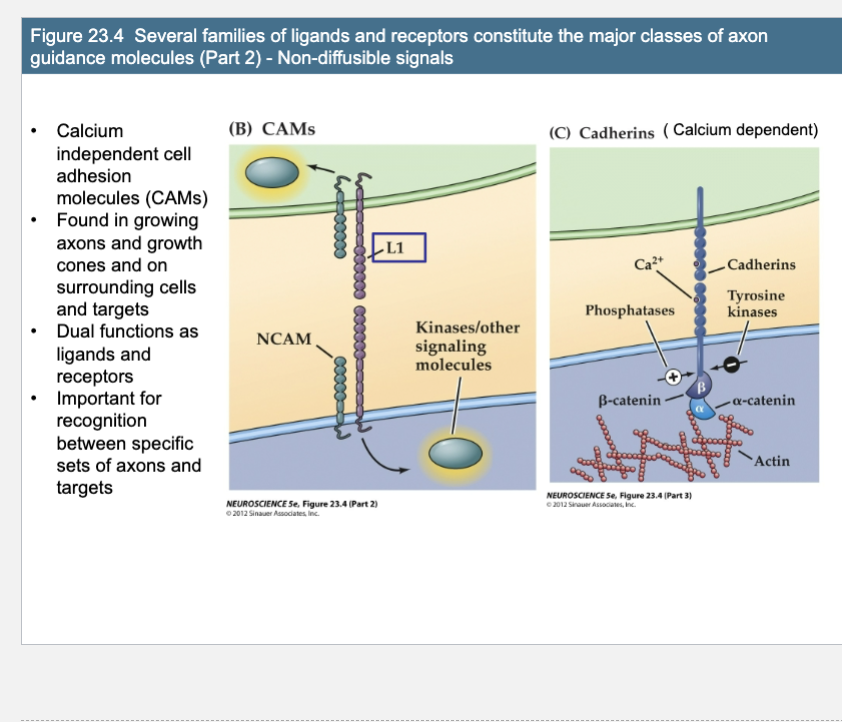
Figure 23.4- Several families of ligands and receptors constitute the major classes of axon
guidance molecules (Part 1) – Non-diffusible signals
CAMs (Ca2+ independent) & Cadherins (Ca2+ dependent)
Calcium independent cell adhesion molecules (CAMs)
Found in growing axons and growth cones and on surrounding cells and targets
Dual functions as ligands and receptors
Important for recognition between specific sets of axons and targets (Calcium dependent)
(B) Homophilic, Ca2+-independent cell adhesion molecules (CAMs) are at once ligands and receptors. Homophilic binding activates intracellular kinases, leading to cytoskeletal changes. → They signal via activation of â-catenin, which influences gene expression
(C) Ca2+-dependent adhesion molecules, or cadherins, are also capable of homophilic binding
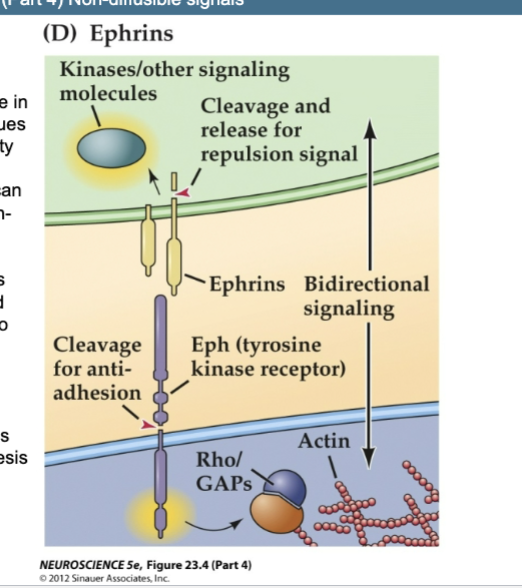
Figure 23.4- Several families of ligands and receptors constitute the major classes of axon
guidance molecules (Part 1) – Non-diffusible signals
Ephrins
Cell- cell recognition code in a variety of tissues
Activate a variety of signaling pathways and can be either growth- promoting or growth-limiting
Immature axons use ephrins and Eph receptors to recognize appropriate pathways for growth and appropriate sites for synaptogenesis
Human developmental disorders (handout)
D) Ephrins, which can be either transmembrane or membrane-associated, signal via the Eph receptors, which are receptor tyrosine kinases.
CLINICAL APPLICATIONS Axon Guidance Disorders
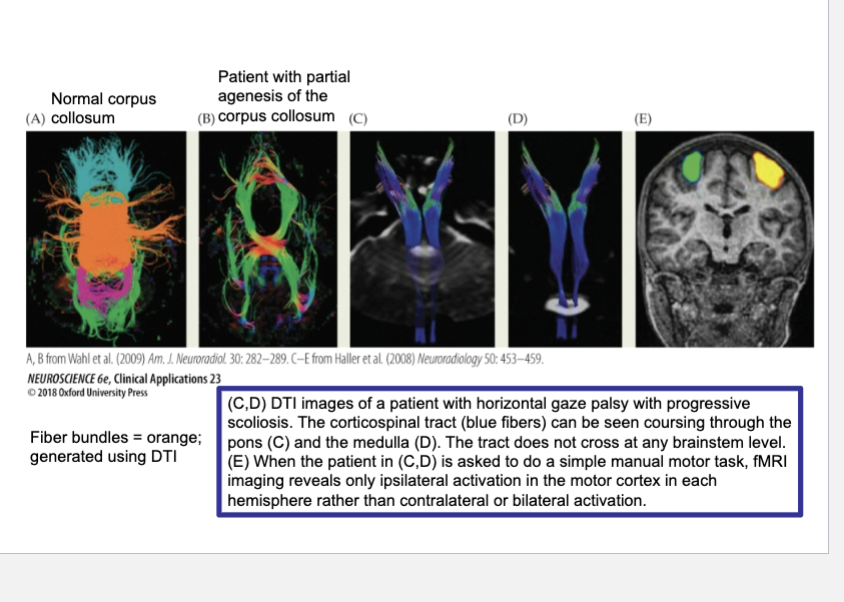
Diffusion tensor imaging (DTI) of a typical human compared with patients suffering from pathologies of axonal guidance. DTI is a refinement of fMRI that applies algorithms to estimate the diffusion direction of water molecules in each voxel imaged.
Because axon bundles tend to constrain the diffusion of water molecules in a single direction, DTI can identify major axon tracts.
(A) In the control individual, the corpus callosum is seen as a corona of fibers crossing the midline (orange).
(B) In a patient with partial agenesis of the corpus callosum, only a few fiber bundles cross the midline.
(C,D) DTI images of a patient with horizontal gaze palsy with progressive scoliosis. The corticospinal tract (blue fibers) can be seen coursing through the pons (C) and the medulla (D). The tract does not cross at any brainstem level.
(E) When the patient in (C,D) is asked to do a simple manual motor task, fMRI imaging reveals only ipsilateral activation in the motor cortex in each hemisphere rather than contralateral or bilateral activation.
L1 Syndrome (CRASH, MASA)
Mutant Gene:
L1 (X-linked)
Horizontal Gaze Palsy with progressive scholiosis
Mutant Gene:
ROBO3
Target derived signals, released by the target cells themselves…
selectively attract growth cones to useful destinations or discourage axon growth toward inappropriate regions
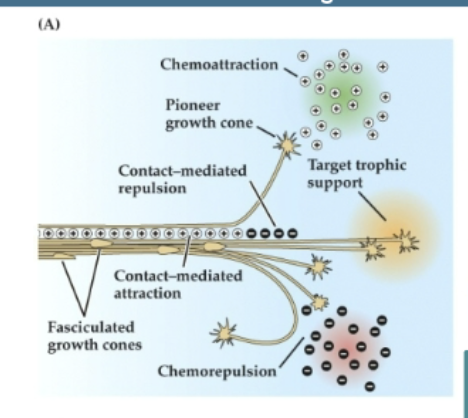
Figure 23.5 Signals provided by the embryonic environment – Diffusible signals (semaphorins)
Figure A
(A) Basic signal classes. Chemoattractant, or tropic, signals (pluses) can operate from a distance and reorient growth toward the source of the cue, often by acting on a pioneer growth cone that sets out a course distinct from that of the fasciculated followers. Similar cues acting on or near the surface of the axon shaft help maintain groups of axons as fascicles, which is essential for formation of coherent nerves and tracts. Chemorepellant signals (minuses) can also act from a distance, or they act at regions where axons must defasciculate from a nascent nerve in order to change their trajectory or avoid an inappropriate target. Trophic signals (gold) support growth and differentiation of the axon and its parent nerve cell.
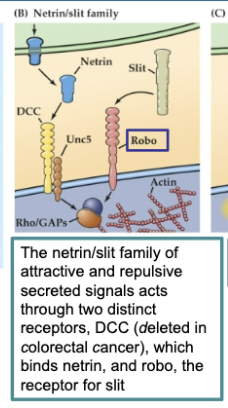
Figure 23.5 Signals provided by the embryonic environment – Diffusible signals (semaphorins)
Figure B
The netrin/slit family of attractive and repulsive secreted signals acts through two distinct receptors, DCC (deleted in colorectal cancer), which binds netrin, and robo, the receptor for slit
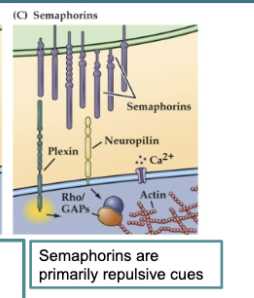
Figure 23.5 Signals provided by the embryonic environment – Diffusible signals (semaphorins)
Figure C
Semaphorins are primarily repulsive cues that can either be bound to the cell surface or secreted. Their receptors (the plexins and neuropilin) are found on growth cones.
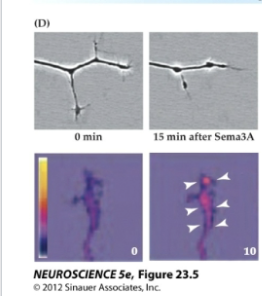
Figure 23.5 Signals provided by the embryonic environment – Diffusible signals (semaphorins)
Figure D
Growth cone–collapsing activity of semaphorin (upper panels) depends on Ca2+ signaling, which is initiated by semaphorin signaling
Chemoattraction and Chemorepulsion
Processes where target-derived signals attract growth cones to useful destinations or discourage axon growth toward inappropriate regions.
Semaphorins
Primarily repulsive cues that can also act as non-diffusible signals in axon guidance. Growth cone collapsing action of semaphorin is dependent on calcium signaling
Once an axon reaches its target region…
additional cell-cell interactions dictate which target cells to innervate from among a variety of potential synaptic partners
Synaptogenesis
The formation of synapses between neurons, involving the recruitment of cytoskeletal elements, localization of calcium channels, and neurotransmitter receptors.
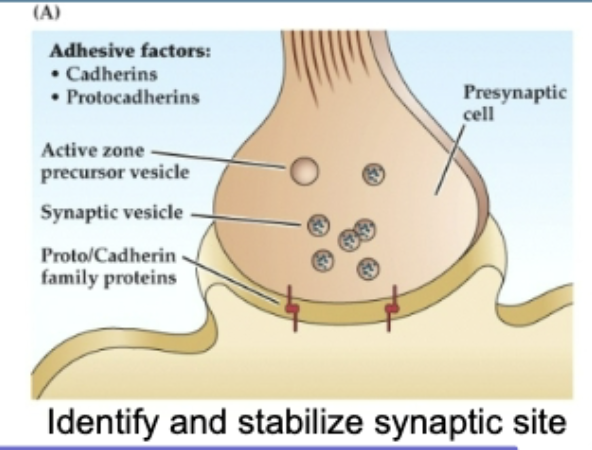
Figure 23.10 Molecular mechanisms involved in synapse formation- Synapatogenesis
Figure A
(A) Synapse Initiation and Stabilization
Cadherins and protocadherins (calcium-dependent adhesion molecules) allow the presynaptic and postsynaptic membranes to recognize each other.
This triggers the initial formation of the active zone in the presynaptic terminal, where synaptic vesicles begin to accumulate.
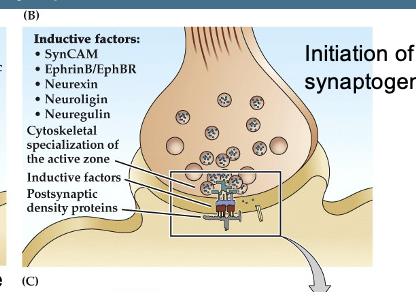
Figure 23.10 Molecular mechanisms involved in synapse formation- Synapatogenesis
Figure B
(B) Synaptogenesis Begins
After initial contact, additional molecules like SynCAM, neurexin, neuroligin, and ephrins are recruited.
These molecules strengthen the adhesion between the two cells.
The presynaptic terminal releases molecules like neuregulin, which help cluster neurotransmitter receptors on the postsynaptic side.
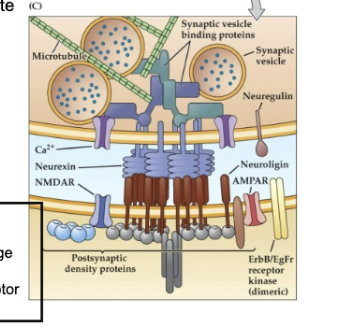
Figure 23.10 Molecular mechanisms involved in synapse formation- Synapatogenesis
Figure C
(C) Mature Synapse Structure and Signaling
Neurexin (presynaptic) binds to neuroligin (postsynaptic) to:
Recruit cytoskeletal elements that position synaptic vesicles.
Localize voltage-gated Ca²⁺ channels for vesicle release.
Help cluster neurotransmitter receptors (like NMDA, AMPA) on the postsynaptic membrane.
Neuregulin is cleaved and binds to ErbB or ErbB/EGF receptors, influencing postsynaptic receptor expression.
Disruptions in Nrg1 (neuregulin) gene are linked to schizophrenia
Recruitment of cytoskeletal elements that localize synaptic vesicles to the presynaptic terminal
Localization of voltage-gated calcium channels
Localization of neurotransmitter receptors
Polymorphisms in the Nrg1 (neuroregulin) gene increase susceptibility to schizophrenia
Neuregulin is released via local proteolytic cleavage and binds to dimeric ErbB receptor kinases or to dimeric ErbB/epidermal growth factor (EGF) receptor kinases
Once synaptic contacts are established…
neurons become dependent on the presence of their targets for continued survival as well as further growth and differentiation of axons and dendrites
Trophic interaction
long term dependency between neurons and their targets
Neurotrophic Factors (Neurotrophins)
Secreted by target cells
Without trophic factors, the neuron will die or undergo apoptosis
During development, a surplus of cells are produced
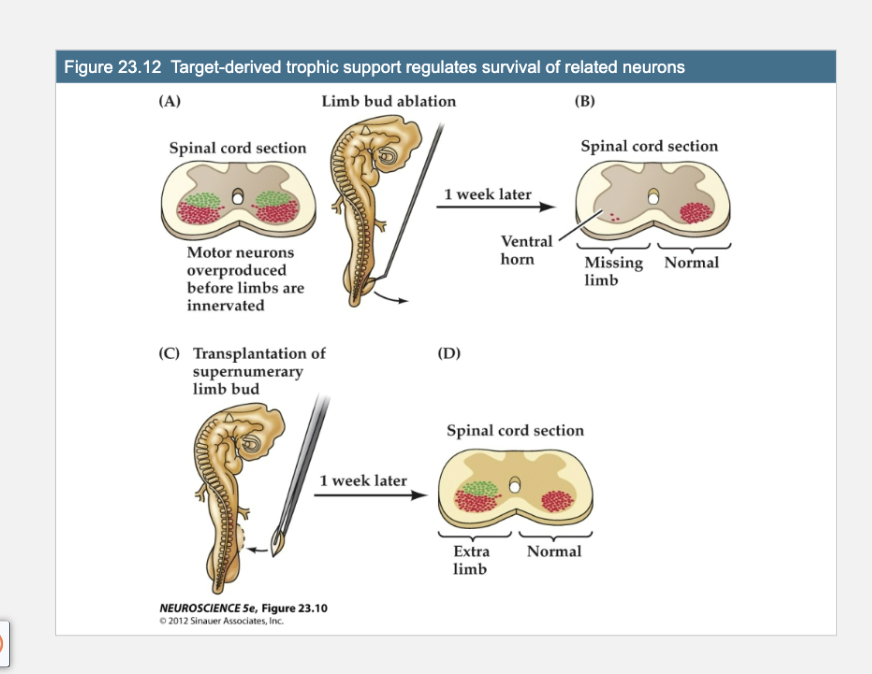
Figure 23.12- Target-derived trophic support regulates survival of related neurons
Figure A
The chick spinal cord generates an excess of neurons (green) prior to the differentiation and innervation of the limb. Normally, some of these neurons are lost once the appropriate level of innervation is established in the developing limb bud. Limb bud amputation in a chick embryo at the appropriate stage of incubation (about 2.5 days) further depletes the pool of motor neurons that would have innervated the missing extremity.
Key Concept:
Motor neurons in the spinal cord are initially overproduced, but only those that successfully connect with a target (e.g., a limb) survive. The target provides trophic (survival) signals like neurotrophins that keep those neurons alive.
Panels A: Limb Bud Ablation
(A)
This shows the normal early development of motor neurons.
Motor neurons are overproduced in the spinal cord (red and green clusters).
They grow out toward the developing limbs, which they will innervate.
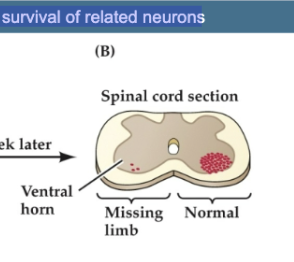
Figure 23.12- Target-derived trophic support regulates survival of related neurons
Figure B
Cross section of the lumbar spinal cord in an embryo that underwent this surgery about a week earlier. The motor neurons (red dots) in the ventral horn that would have innervated the hindlimb have degenerated almost completely. A normal complement of motor neurons is present on the other side; most of the normally supernumerary neurons have been lost.
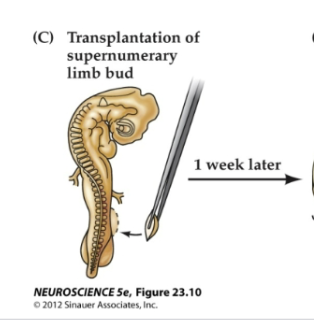
Figure 23.12- Target-derived trophic support regulates survival of related neurons
Figure C
Adding an extra limb bud before the normal period of cell death rescues early-generated neurons that normally would have died.
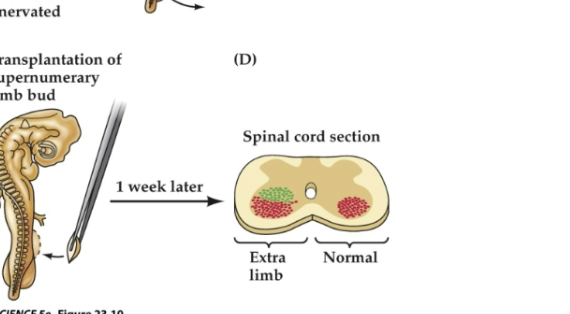
Figure 23.12- Target-derived trophic support regulates survival of related neurons
Figure D
Such augmentation leads to an abnormally large number of limb motor neurons on the side related to the extra limb, and these neurons are recruited from the pool of cells overproduced at an earlier stage of development (green dots) rather than generated de novo through cell proliferation elicited by the added target.
Trophic Interaction
Long-term dependency between neurons and their targets, regulated by neurotrophic factors.
The mature wiring plan of the CNS is a result of a flexible process in which…
Local neuronal connections are formed, removed, and remodeled according to the molecular constituents in the environment, the structure and size of the target, and ongoing electrical activity
The mature wiring plan of the CNS ensures…
that every target cell is innervated and continues to be innervated by the correct number of inputs and synapses and that every axon contacts the right number of target cells
Convergence
The number of inputs to a target cell.
Divergence
The number of connections made by a neuron.
Neurotrophic interactions regulate…
convergence and divergence; this is also influenced by the size and shape of neurons, particularly the dendrites
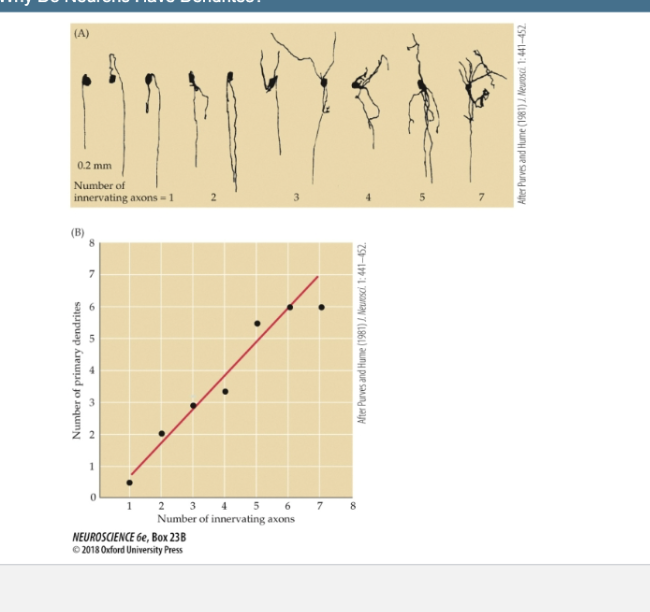
Box 23 B- Why do neurons have dendrites
(A) Axons innervating ciliary ganglion cells in adult rabbits. Neurons studied electrophysiologically and then labeled by intracellular injection of a marker enzyme have been arranged in order of increasing dendritic complexity. The number of axons innervating each neuron is indicated.
(B) This graph summarizes observations of the number of innervating axons on a large number of rabbit ciliary ganglion cells. There is a strong correlation between dendritic geometry and input number.
3 Functions of trophic factors in the formation of mature neural circuits
Survival of a subset of neurons from a larger population
Formation and maintenance of appropriate numbers of
connectionsElaboration of axonal and dendritic branches to support these
connections
Neurons and their targets:
Neurons depend on the availability of some minimum amount of
trophic factorTarget tissues synthesize appropriate trophic factors and make
them available to neuronsThere is interneuronal competition for available trophic factors;
they are produced in limited amounts
NGF (Nerve Growth Factor)
1st trophic factor discovered. Involved in elaboration of axonal and dendritic branches.
Other Neurotrophins: BDNF (Brain-Derived Neurotrophic Factor)
A neurotrophin that supports the survival and differentiation of neurons.
NT-3, NT-4/5
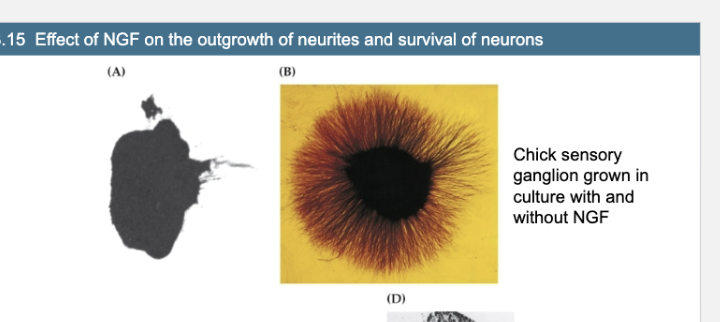
Figure 23.15- Effect of NGF on the outgrowth of neurites and survival of neurons
Figure A vs Fig B
Chick sensory ganglion grown in culture without NGF
Chick sensory ganglion grown in culture with NGF
NGF stimulates a halo of neurite outgrowth from the ganglion cells.
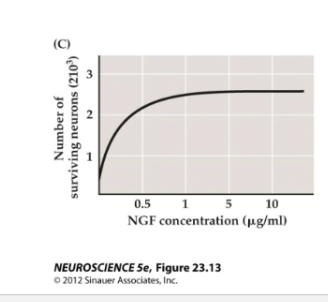
Figure 23.15- Effect of NGF on the outgrowth of neurites and survival of neurons
Figure C
NGF influences the survival of newborn rat sympathetic ganglion cells grown in culture for 30 days. Dose–response curves confirm the dependence of these neurons on the availability of NGF.
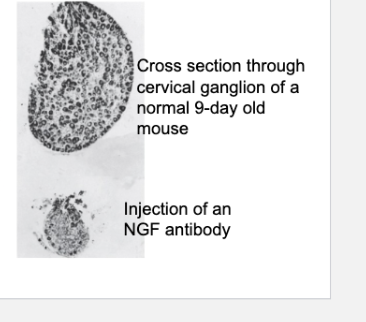
Cross section of a superior cervical ganglion from a normal 9-day-old mouse (top) compared with a similar section from a littermate that was injected daily since birth with NGF antiserum (bottom). The ganglion of the treated mouse shows marked atrophy, with obvious loss of nerve cells.
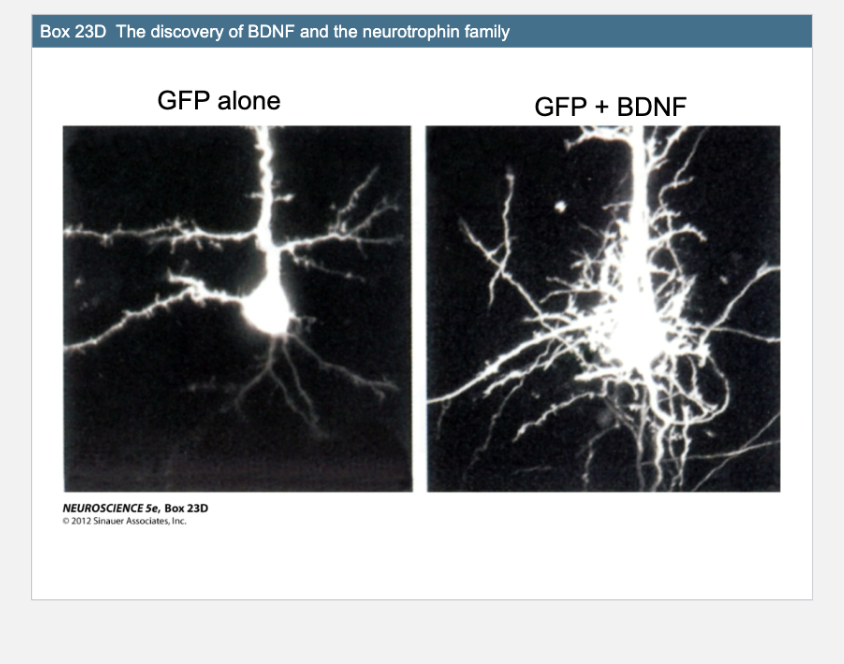
Box 23D- BDNF
Left image (GFP alone): A neuron expressing GFP (green fluorescent protein) without brain-derived neurotrophic factor (BDNF). It shows normal, limited branching.
Right image (GFP + BDNF): A neuron expressing GFP and treated with BDNF. It shows extensive dendritic branching and more complex arborization.
BDNF (Brain-Derived Neurotrophic Factor) is a…
neurotrophin that enhances neuronal growth and branch complexity.
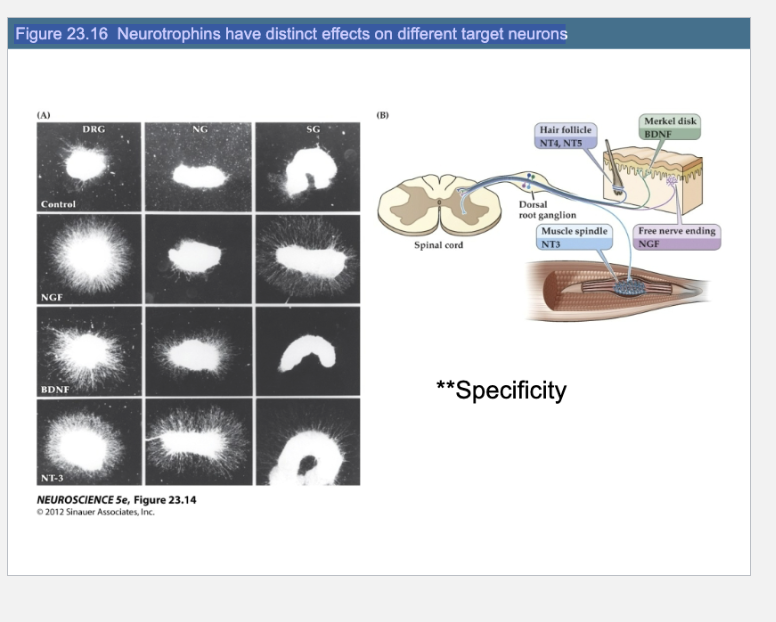
Figure 23.16 Neurotrophins have distinct effects on different target neurons
What does Figure 23.16 show about neurotrophin specificity?
It shows that different neurotrophins (NGF, BDNF, NT-3) selectively promote neurite outgrowth in specific ganglia types. For example, NGF promotes outgrowth in DRG and sympathetic ganglia but not in nodose ganglia. In vivo, distinct sensory receptors like Merkel disks or free nerve endings depend on specific neurotrophins (e.g., BDNF or NGF) from their target tissues for development and survival.
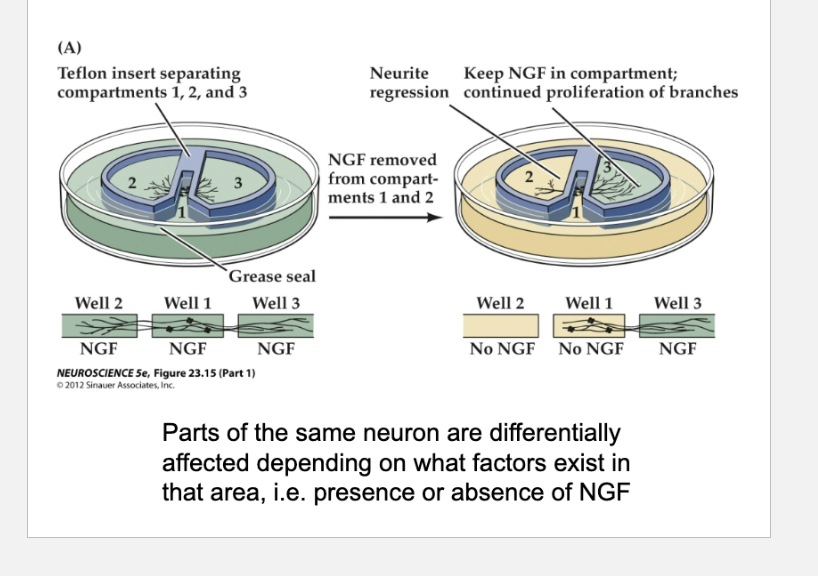
What does Figure 23.17 show about how neurotrophins influence neurite growth?
Parts of the same neuron are differentially affected depending on what factors exist in that area, i.e. presence or absence of NGF
A: It shows that neurotrophins like NGF can act locally—neurites only grow in areas where NGF is present, and withdraw from areas where it is removed. Neurotrophins also trigger local calcium signals in growth cones and form signaling endosomes that travel to the cell body to activate growth-related pathways and gene expression.
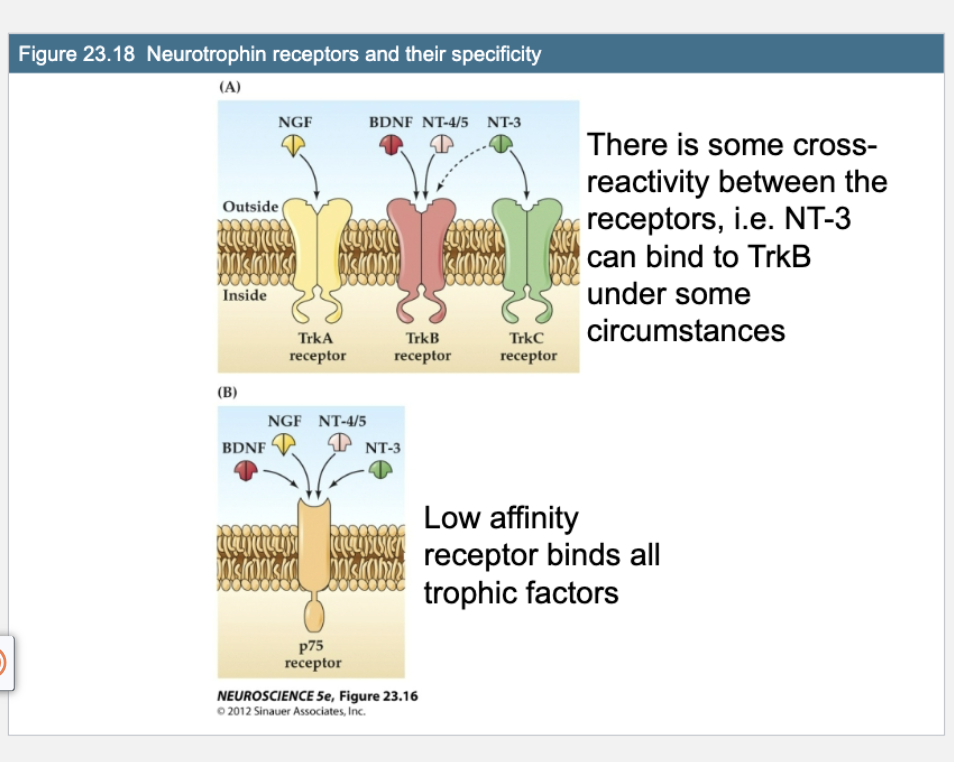
Q: What does Figure 23.18 show about neurotrophin receptor specificity?
A: Each Trk receptor binds specific neurotrophins: TrkA binds NGF, TrkB binds BDNF and NT-4/5, and TrkC binds NT-3. There’s some cross-reactivity (e.g., NT-3 can also bind TrkB). The p75 receptor is a low-affinity receptor that can bind all neurotrophins.
Trk receptors (high-affinity, specific)
TrkA → binds ?
TrkB → binds ? and ?
TrkC → binds ?
TrkA → binds NGF
TrkB → binds BDNF and NT-4/5
TrkC → binds NT-3
p75 receptor (low-affinity, broad)
Binds all ? with low specificity
Can promote survival, neurite growth, OR cell death depending on the context
Binds all neurotrophins with low specificity
Can promote survival, neurite growth, OR cell death depending on the context
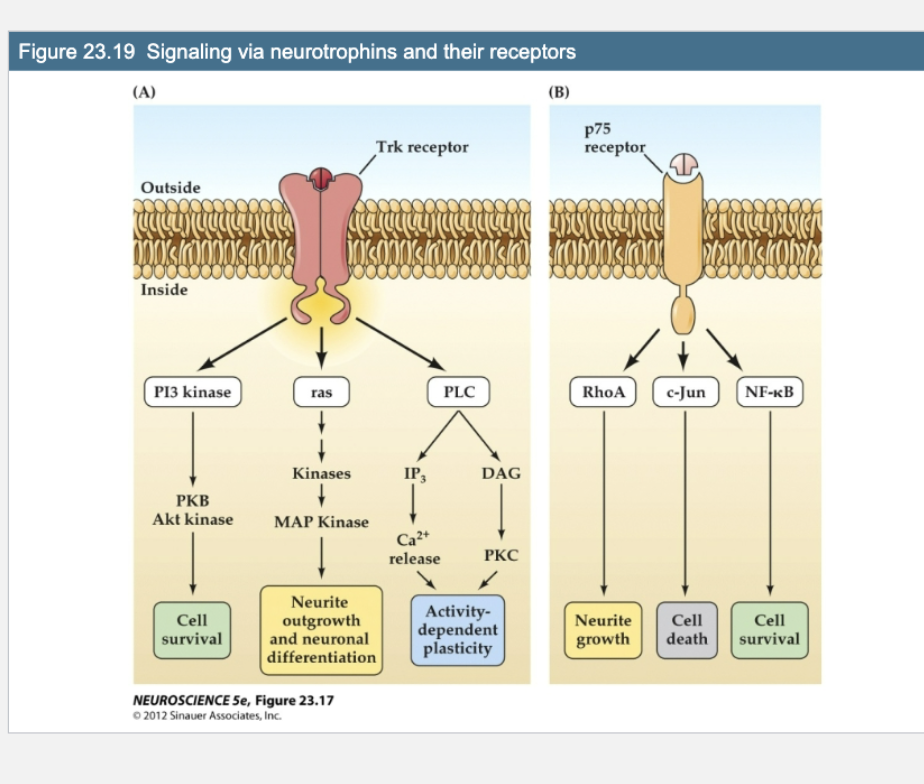
Figure 23.19- What do Trk and p75 receptors trigger when activated by neurotrophins?
Trk receptors activate:
PI3K/Akt → Cell survival
Ras/MAPK → Neurite growth & differentiation
PLC/IP₃/DAG → Activity-dependent plasticity
p75 receptor can activate:
RhoA → Neurite growth
c-Jun → Cell death
NF-κB → Cell survival
Trk receptor signaling leads to:
Cell survival (via Akt/PKC)
Neurite outgrowth (via MAPK pathway)
Synaptic plasticity (via Ca²⁺/PKC pathway)
p75 signaling can:
Promote growth via Rho kinases
Cause cell cycle arrest or apoptosis via other pathways
1. How does disruption of Par3 function affect the differentiation of a neuron?
Disruption of Par-3 prevents a neuron from polarizing properly. None of the neurites elongate into a defined axon, and the axon-specific marker Tau is incorrectly expressed in all processes instead of just one. This means the neuron cannot establish a proper axon-dendrite identity
2A. Describe the function of a growth cone.
A growth cone is a specialized structure at the tip of a growing neurite. It explores the environment, senses guidance cues, and directs the neurite’s extension toward appropriate synaptic targets
2B. What part of the cytoskeleton is localized in the growth cone?
Actin filaments are concentrated in the lamellipodium and filopodia for directional movement.
Microtubules extend from the axon shaft into the base of the growth cone, providing structural support and transport
3. Describe one event that happens during the establishment of the synapse.
During synaptogenesis, molecules like cadherins, neurexins, and neuroligins help identify and stabilize synaptic sites. Cytoskeletal elements are recruited to localize synaptic vesicles and neurotransmitter receptors at the presynaptic and postsynaptic terminals
4. What effects do neurotrophins have on neurons?
Neurotrophins:
Support neuron survival (e.g., NGF via TrkA).
Promote neurite outgrowth and branching.
Influence synaptic connectivity and plasticity.
Act locally and signal back to the nucleus to affect gene expression.
Without them, many neurons undergo apoptosis during development.