Unit Conversions (Lecture 4)
0.0(0)
0.0(0)
Card Sorting
1/13
There's no tags or description
Looks like no tags are added yet.
Study Analytics
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
14 Terms
1
New cards
From Farenheit to Celsius
(Farenheit *-32)/1.8
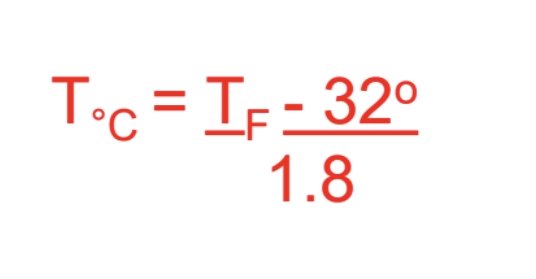
2
New cards
From Celsius to Farenheit
1.8*Celius+32

3
New cards
From Celsius to Kelvin
Celsius +273.15
4
New cards
From Kelvin to Fahrenheit
(1.8*Kelvin)-459.67
5
New cards
1 kilogram is
2.2 pounds
6
New cards
1 inch is
2.54 cm
7
New cards
The new concentration (of the solution) can be calculated using the formula:
Final concentration = [Original conc.] [dilution(s) done]
8
New cards
Dilution factor formula
Total volume/ volume from OG
9
New cards
Conc. of original sample =
(result obtained) (dilution factor)
10
New cards
Dilution Ratio
eg.1/10 dilution of a 1/5 stock
11
New cards
Percent:
as parts per 100 parts
12
New cards
mg/dL
it is a weight/volume expression (mg per 100 mL)
13
New cards
Molarity(M)
# of moles of solute per 1L of solution
14
New cards
Normality(N)
# of equivalents of solute per 1L of solution