Exam 2
1/156
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
157 Terms
List the Passive transport processes
Simple diffusion, Facilitated diffusion, Osmosis
List the Active transport processes
Primary active transport, Secondary active transport, Bulk/Vesicular transport, Exocytosis, Endocytosis
Explain the protein, vesicle, and energy requirements of Simple diffusion
Does not involve any
Explain the protein, vesicle, and energy requirements of Facilitated diffusion
Requires a transport protein.
What is a channel protein
A small protein that helps facilitated diffusion. No change
What is a carrier protein
A large protein that helps facilitated diffusion. 2 shape changes
Explain the protein, vesicle, and energy requirements of Osmosis
Requires movement of water
Explain the protein, vesicle, and energy requirements of Primary active transport
Uses a protein called a pump embedded in the cell membrane. Requires ATP as energy
Explain the protein, vesicle, and energy requirements of Secondary active transport
Requires concentration of solute A to move up solute B’s concentration gradient. Energy comes from the electrochemical gradient of a molecule like Sodium NA+
Explain the protein, vesicle, and energy requirements of Vesicular transport
Requires vesicles that carry proteins. ATP hydrolysis provides the energy for the entire process.
Explain the protein, vesicle, and energy requirements of Exocytosis
Vesicle fuses with plasma membrane and releases contents. Requires ATP for energy
Explain the protein, vesicle, and energy requirements of Endocytosis
Vesicle leaves the plasma membrane and takes contents. Requires ATP for energy
What is pinocytosis and its requirements.
Form of endocytosis. “Cell drinking”. Random uptake of extracellular materials in small vesicles. essentially the same as endocytosis except smaller. requires ATP
What is receptor-mediated endocytosis and its requirements
Form of endocytosis. Uses more receptor proteins to carry large particles across the cell membrane. cells pick certain materials in increase incorporation. requires ATP
Explain the protein, vesicle, and energy requirements of Phagocytosis
only certain cells. Form of endocytosis. AMEOBA. Engulfs large particles into a type of vesicle called a phagosome. Requires ATP for energy
What materials are transported by simple diffusion.
oxygen, carbon dioxide, small non-polar molecules, and certain lipids
What materials are transported by facilitated diffusion
Polar and charged molecules like amino acids, carbohydrates, ions, and nucleosides
What materials are transported by osmosis
Water
What materials are transported by primary active transport
Ions like sodium, potassium, calcium, and hydrogen
What materials are transported by secondary active transport
glucose, amino acids, and certain ions like hydrogen ions (protons)
What materials are transported by vesicular transport
large molecules like proteins, lipids, hormones, and even large particles like bacteria or cellular debris within a cell
What materials are transported by exocytosis
hormones, neurotransmitters, digestive enzymes, proteins, waste products, toxins, and certain components of the extracellular matrix
What materials are transported by endocytosis
nutrients, signaling molecules, pathogens, and even parts of the plasma membrane
What materials are transported by pinocytosis
extracellular fluid and dissolved solutes such as small molecules like vitamins, ions, fats (like low-density lipoprotein), and antigens
What materials are transported by receptor-mediated endocytosis
low-density lipoprotein (LDL) cholesterol, iron bound to transferrin, hormones, growth factors, vitamins, viruses, and other proteins and macromolecules
What materials are transported by Phagocytosis
bacteria, dead cells, cellular debris, and other foreign substances
How do lipids with hydrophilic and hydrophobic regions behave in an aqueous environment?
the polar hydrophilic head group interacts with the polar water molecules. the nonpolar hydrophobic tail does not interact with water but other nonpolar tail groups or hydrophobic molecules.
Can you predict which type of fat contains saturated fatty acids and which type contains unsaturated fatty acids?
saturated fatty acids tend to be solid at room temperature, and unsaturated fatty acids tend to be liquid. Butter is solid, many plant oils are liquid
What are two ways in which proteins associate with membranes?
integral membrane proteins are permanently associated with the membrane and cannot be removed.
peripheral membrane proteins are temporarily associated with the membrane and can easily be experimentally separated.
What would happen in the FRAP experiment if proteins did not move in the plane of a membrane?
FRAP is photobleaching. First, the proteins in the cell membrane are dyed. A laser is then used to bleach a small area. then, colored should move into that area. if they don’t, the area will stay bleached
What are the roles of lipids and proteins in maintaining the selective permeability of membranes?
Lipids help maintain the selective permeability of the membrane by stopping charged molecules/ions from diffusing freely. Proteins transport larger molecules by acting as channels and carriers
A container is divided into two compartments by a membrane that is fully permeable to water and small ions. Water is added to one side of the membrane (side A), and a 5% solution of sodium chloride (NaCl) is added to the other side (side B). In which direction will water molecules move? In which direction will sodium and chloride ions move? When the concentration is equal on both sides, will movement stop?
water molecules move in both directions, movement of water molecules is from side A to side B. Water moves from regions of higher water concentration to regions of lower water concentration. sodium and chloride ions move in both directions, but they move from side A to B because of diffusion.
Movement still happens when concentration is equal
What is the difference between passive and active transport?
passive transport moves cells in/out through diffusion when there is a concentration difference. Molecule moves from high to low concentration
active transport moves molecule in/out of cells against its concentration gradient. Molecules move through transport proteins and need energy
In the absence of the sodium–potassium pump, the extracellular solution becomes hypotonic relative to the inside of the cell. Poisons such as ouabain can interfere with the action of the sodium–potassium pump. What are the consequences for the cell?
if the sodium–potassium pump is made inactive, the cell will swell and burst, as the intracellular fluid becomes hypertonic relative to the outside of the cell and water moves into the cell by osmosis.
What are three different ways in which cells maintain size and shape?
Cells can use active transport to maintain the intracellular solute concentration
The cell walls of plants, fungi, and bacteria help maintain the cell’s size and shape
the cytoskeleton is a network of proteins inside of cells that helps maintain the shape of many cells.
Membrane functions
Defines cell, separates cell, Transport across membrane, communication, attachment
Membrane structure
Drawing
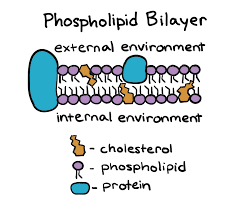
Lipid bilayer
Separates inside of the cell from the outside
Lipid bilayer phospholipid function
Allows the cell to be selectively permeable
What kind of molecule is a phospholipid
Amphipathic molecule
What is a Phospholipids behavior in water
They can form either a bilayer, micelle, or a liposome
how do the fatty acid tails affect fluidity
unsaturated fatty acid tails promote fluidity due to their "kinks" which prevent tight packing, the degree of saturation in the fatty acid tails determines membrane fluidity.
What is the job of cholesterol, ergosterol, and phytosterol
these help in maintaining fluidity, structure, and permeability through the plasma membrane
What are glycolipids
Amphipathic lipids with a carbohydrate attached.
what do glycolipids do in the plasma membrane
stabilize the plasma membrane, help cells recognize each other, and facilitate cell signaling
What is the movement of lipids in the plasma membrane
rotational, lateral, flip flop between monolayers
What is distribution of lipids in membrane (lipid rafts)
aggregations of lipids in a membrane. (membrane function)
What is an integral protein
a protein deeply embedded in membrane. may need to dissolve membrane to isolate protein.
what is a transmembrane protein
a protein that extends through the lipid bilayer and pokes out of both sides. extracellular and cytoplasmic
what is a peripheral protein
a protein that is loosely associated with the membrane. can be extracted using certain salt solutions in a certain PH
what are movements of proteins in the membrane
Laterally, and rotationally. CAN NOT FLIP FLOP
where are carbohydrates located
extracellular surface of a plasma membrane attached to a lipid or a protein
what is a glycolipid
lipid + carbohydrate
what is a glycoprotein
protein + carbohydrate
what is a glycolax or sugar coat
all external carbohydrates on an animal cell’s surface
what is membrane Asymmetry
when the plasma membrane is uneven in terms of proteins, lipids, etc
What are the ways that organisms obtain energy and carbon from the environment? what are the names
Phototrophs get sunlight energy, Chemotrophs get energy from chemical compounds. Autoptrophs get energy from inorganic sources, heterotrophs get carbon from organic compounds.
What is the difference between catabolism and anabolism?
Catabolism breaks down macromolecules into ATP. Anabolism builds macromolecules and needs energy like ATP
What are two basic forms of energy? Provide an example of each.
One basic form of energy is kinetic energy. flexing a muscle and throwing a ball. The other basic form of energy is potential energy. A ball sitting on the top of the stairs.
What is the relationship between strength of covalent bond and the amount of chemical energy it contains?
The stronger the covalent bond, the less chemical energy it contains. The weaker the covalent bond, the more chemical energy it contains.
How do proteins, lipids, and carbohydrates get good sources of chemical energy
proteins have many carbon carbon and carbon hydrogen bonds. These bonds are relatively weak‒ ‒and are therefore rich sources of chemical energy.
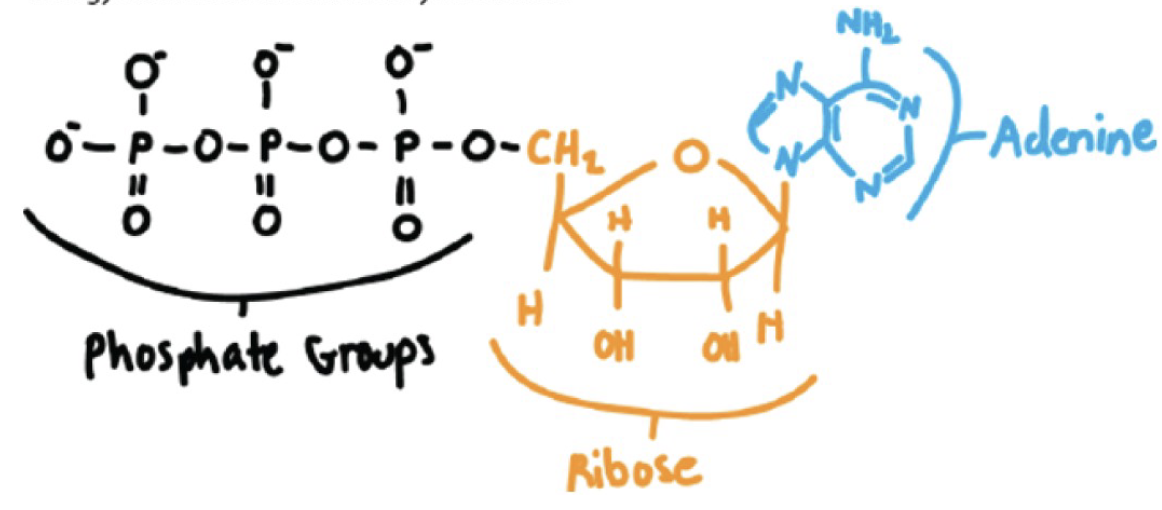
Draw the structure of ATP, indicating the bonds that are broken to power the cell.
Drawing
what is the first law of thermodynamics
The first law of thermodynamics is the law of conservation of energy. It says energy can’t be created or destroyed, only transformed.
What is the second law of thermodynamics
The second law of thermodynamics energy is transformed with universal disorder. this is entropy. amount of energy stays the same, but some of it is transformed to heat (entropy)
Cold air has less entropy than hot air. The second law of thermodynamics states that entropy always increases. Do air conditioners violate this law?
No, because the second law of thermodynamics applies to the universe as a whole. we have to think about the heat released to the outside too. AC produces more hot air than cold air, and therefore total entropy increases,
For a chemical reaction with a positive value of ΔG, what are the possible relative values for ΔH and TΔS? Is the reaction spontaneous or not?
If the enthalpy difference (ΔH) is positive and the entropy difference (ΔS) is negative, then ΔG is positive. This kind of reaction is endergonic and nonspontaneous.
For a chemical reaction with a negative value of ΔG, what are the possible relative values for ΔH and TΔS? Is the reaction spontaneous or not?
if the enthalpy difference (ΔH) is negative and the entropy difference (ΔS) is positive, then ΔG is negative. This kind of reaction is exergonic and occurs spontaneously.
What is Gibbs free energy (G)
The amount of energy available to do work is called Gibbs free energy (G). compare the free energy of the reactants and products to determine whether there is energy available to do work. This difference is called ΔG. ΔG = ΔH ‒ TΔS.
How does increasing the temperature affect the change in free energy (ΔG) of a chemical reaction?
Increasing the temperature increases the value of T∆S, which decreases ∆G, since ∆G = ∆H – T∆S. An increase in temperature makes it more likely that a reaction will proceed without a net input of energy
How can the hydrolysis of ATP drive nonspontaneous reactions in a cell?
The hydrolysis of ATP releases energy. This energy can be used to drive nonspontaneous reactions in a cell if the total ΔG for the entire pathway is negative.
What are three characteristics of enzymes, and how does each permit chemical reactions to occur in cells?
Enzymes reduce the activation energy of a chemical reaction by stabilizing the transition state and decreasing its free energy.
Enzymes are catalysts that participate in a chemical reaction, forming complexes with products and reactants
Enzymes are also highly specific. They typically catalyze only one reaction, recognizing a specific substrate.
Finally, inhibitors and activators can influence enzyme activity. Inhibitors decrease the activity of enzymes, whereas activators increase this activity.
Which of the following do enzymes change: ΔG, reaction rate, types of product, activation energy, and/or the laws of thermodynamics?
Enzymes increase the reaction rate and decrease the activation energy. Enzymes do not change the other parameters.
How does protein folding allow for enzyme specificity?
shaping the enzyme into a unique three-dimensional structure with a specific active site, allowing it to bind and catalyze reactions with only certain substrates, like a lock and key
what is the first major stage of respiration. Glycolysis
1. Glycolysis: glucose is partially broken down to pyruvate and a modest amount of energy is released to ATP.
what is the second major stage of respiration. pyruvate oxidation.
2. Pyruvate oxidation: pyruvate (the breakdown product of glucose from stage 1) is converted to acetyl-coenzyme A, and carbon dioxide and electron carriers are produced.
What is the third major stage of respiration. citric acid cycle
3. Citric acid cycle: acetyl-CoA is broken down and carbon dioxide, ATP, and reduced, electron carriers are produced.
what is the fourth major stage of respiration. oxidative phosphorylation
4. Oxidative phosphorylation: electron carriers generated in stages 1–3 donate electrons to an electron transport chain. (electrons in series of membrane-associated proteins). in the process, large amounts of ATP are produced
What is an oxidation–reduction reaction?
Oxidation reduction reactions are used to store or release chemical energy.
Why is the breakdown of glucose in the presence of oxygen to produce carbon dioxide and water an example of an oxidation–reduction reaction?
Glucose loses electrons as it breaks down into simpler molecules like carbon dioxide (CO2). This loss of electrons is oxidation.
Oxygen (O2) gains electrons as it combines with hydrogen to form water (H2O). This gain of electrons is reduction.
For each of the following pairs of molecules, indicate which member of the pair is reduced and which is oxidized, and which has more chemical energy and which has less chemical energy: NAD+/NADH; FAD/FADH2; CO2/C6H12O6
The reduced molecules are NADH, FADH2, and C6H12O6, and the oxidized molecules are NAD+, FAD, and CO2. reduced have more chemical energy
What are the two different ways in which ATP is generated in cellular respiration?
ATP is generated by substrate-level phosphorylation(directly transfers a phosphate group to ADP)
and oxidative phosphorylation(energy released from transport chain).
What is the overall chemical equation for glycolysis?
Glucose + 2NAD+ + 2ADP + 2Pi → 2 pyruvate + 2ATP + 2NADH + 2H+ + 2H2O
At the end of glycolysis, but before the subsequent stages in cellular respiration, which molecules contain some of the chemical energy held in the original glucose molecule?
At the end of glycolysis, the energy in the original glucose molecule is contained in pyruvate, ATP, and NADH.
Where does pyruvate oxidation take place?
Pyruvate oxidation is the first stage of cellular respiration that takes place inside the mitochondria. Specifically, it takes place in the mitochondrial matrix
At the end of pyruvate oxidation, but before the subsequent stages of cellular respiration, which molecules contain the energy held in the original glucose molecule?
At the end of pyruvate oxidation, the energy in the original glucose molecule is contained in acetyl- CoA and NADH.
At the end of the citric acid cycle, but before the subsequent stages of cellular respiration, which molecules contain the energy held in the original glucose molecule?
At the end of the citric acid cycle, the energy in the original glucose molecule is contained in ATP, NADH, and FADH
What is the function of running the citric acid cycle in reverse?
generate intermediates in the synthesis of other molecules and to incorporate carbon into organic molecules
Animals breathe in air that contains more oxygen than the air they breathe out. Where is oxygen consumed?
Oxygen is consumed in cellular respiration. Oxygen is the final electron acceptor in the electron transport chain and is converted to water
How does the movement of electrons along the electron transport chain lead to the generation of a proton gradient
The energy from electron movement is harnessed to physically move protons against their concentration gradient, building up a potential energy store that can later be used to generate ATP.
How is a proton gradient used to generate ATP?
the flow of protons down the concentration gradient through ATP synthase provides the energy needed to synthesize ATP.
Uncoupling agents are proteins spanning the inner mitochondrial membrane that allow proteins to pass through the membrane and bypass the channel of ATP synthase. Describe the consequences of uncoupling agents for the proton gradient and ATP production.
Protons in the intermembrane space flow through ATP synthase, which has two parts: Fo (the channel) and F1 (the ATP-making unit). Proton flow causes the enzyme to rotate, converting the proton gradient into mechanical energy, which enables F1 to synthesize ATP from ADP and Pi.
Bread making involves ethanol fermentation and typically uses yeast, sugar, flour, and water. Why are yeast and sugar used?
Yeast cells are eukaryotes. Yeast can use sugar as a food source for ethanol fermentation. The carbon dioxide produced in the process causes the bread to rise. The ethanol is removed in the baking process
What are two different metabolic pathways that pyruvate can enter?
In the first pathway, pyruvate is converted to acetyl-CoA, which enters the citric acid cycle to produce ATP, NADH, and FADH2. The second pathway, fermentation, occurs without oxygen and recycles NADH to NAD+.
What are the two types of fermentation pathways
In lactic acid fermentation, NADH transfers electrons to pyruvate, forming lactic acid. In ethanol fermentation, pyruvate produces acetaldehyde, which is reduced by NADH to form ethanol.
What are three types of organic molecules that contain high potential energy in their bonds and therefore can act as “fuel” molecules?
Carbohydrates, lipids, and proteins are all considered fuel molecules because they have high potential energy in their chemical bonds
What happens to chemical reactions that generate ATP when levels of ATP are high in a cell?
When ATP levels are high, the cell has a high amount of free energy. In this case, pathways that generate ATP are slowed, or down-regulated
How does muscle tissue generate ATP during short-term exercise
Muscle tissue generates ATP during short-term exercise by converting stored glycogen to glucose. Glucose is rapidly broken down anaerobically to pyruvate, which then feeds into the lactic acid fermentation pathway.
How does muscle tissue generate ATP during long-term exercise
In long-term exercise, the liver releases glucose into the blood, which muscles use to produce ATP. Additionally, adipose tissue releases fatty acids that muscles break down through β-oxidation. These processes are slower but produce more ATP than fermentation.
what is the energy flow on earth
Sunlight energy, photosynthesis, chemical energy, respiration, work, heat (lost to universe)
What are the different forms of energy
Kinetic and potential energy