8.1 Introduction to Addition Reactions
1/30
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
31 Terms
Define an addition reaction in alkenes
A reaction where two atoms or groups are added across a C=C double bond, breaking the π bond and forming two new σ bonds
What bond is broken during the addition reaction?
The π bond of the alkene
What type of bond atoms forms after π bond breaks?
Two new σ bonds
How do addition reactions differ from elimination reactions?
Addition adds atoms to a molecule (increasing saturation), while elimination removes atoms (creating unsaturation pr double bonds)
Why are alkenes reactive in addition reactions?
Their π bond is electron-rich and easily attacked by electrophiles
What are the typical reacting species in an alkene addition reaction?
An electrophile (E⁺) attacks the π electrons, followed by a nucleophile (Nu⁻) attacking the carbocation intermediate.
Explain what happens to the carbon hybridization in an addition reaction
Each carbon in the C=C bond changes from sp2 to sp3 hybridization after addition
Predict the saturation level after an addition reaction
The product becomes more saturated (fewer double bonds)
What drives addition reactions energetically?
The formation of strong σ bonds releases more energy than is required to break the π bond.
What is the general mechanism type for most alkene addition reactions?
Electrophilic addition mechanism
Why is the π bond weaker than a σ bond?
π bonds result from side-to-side overlap of p-orbitals, which is less effective than the end-to-end overlap in σ bonds.
What happens to molecular geometry after addition?
The double bond’s planar geometry becomes tetrahedral around each carbon
If asked to identify the type of reaction that converts an alkene to an alkane, what should you answer?
Hydrogenation- an addition reaction with H2
Explain in one sentence why π bonds act as nucleophiles.
Because they contain loosely held electrons that attack electrophilic centers
On a reaction coordinate diagram, where is the π bond breaking and new σ bonds forming?
At the transition state during electrophilic addition
Why are alkenes considered versatile in organic synthesis?
Because they undergo many different addition reactions, forming a wide variety of functional groups
What part of the alkene gives it its reactivity?
The π bond between the two sp²-hybridized carbons
What are the two main ways a π bond can behave in reactions?
As a weak base or as a weak nucleophile
When does a π bond act as a weak base?
When it reacts with a strong acid (protonation by H+ )
When does a π bond act as a weak nucleophile
When it donates electrons to attack an electrophile (E⁺)
What happens when a π bond is protonated by a strong acid?
A carbocation intermediate forms on one of the carbons of the former double bond
What does the π bond attack during electrophilic addition reactions?
An electrophile (E⁺), such as H⁺ or a polarized atom in a molecule like H–Br
Why can π bonds function as electron donors?
Because their electrons are loosely held and can be shared or donated to electrophiles
What is the first step in most electrophilic addition reactions of alkenes
Protonation or electrophilic attack on the π bond, forming a carbocation
What structural change occurs in the alkene after π bond attack?
The double bond breaks, forming a new σ bond and a carbocation center
Why do π bonds react easily with acids or electrophiles?
They are high in energy and easily polarized, making them reactive toward electron-poor species
What type of reactions will repeatedly show π bond protonation or electrophilic attack?
Electrophilic addition reactions throughout alkene chemistry
How does π bond reactivity make alkenes useful synthetic precursors?
It allows chemists to transform them into alcohols, halides, diols, or other functional groups by selecting specific reagents
What hybridization change occurs when a π bond reacts?
The sp² carbons become sp³ as the π bond converts to σ bonds
If asked, “Why are π bonds both weak bases and weak nucleophiles?” what’s the short answer?
Because they contain electron density that can either accept a proton (basic behavior) or attack an electrophile (nucleophilic behavior)
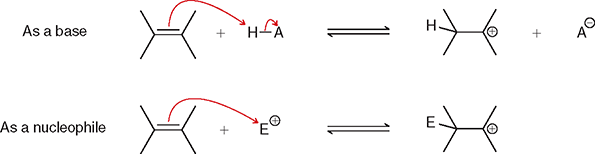
What is happening in this picture?
The first process illustrates that 𝜋 bonds can be protonated by a strong acid
The second process illustrates that 𝜋 bonds can attack electrophiles.