Lipid metabolism
1/38
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
39 Terms
Fat percentage of daily energy
40% of daily energy
~80-100 grams per day
Three types of dietary lipids
Triglycerides (95%) → most important
Phospholipids (2%)
Sterols (3%)
Structure triglycerides
Three fatty acids linked via a glycerol molecule
Can be different fatty acids
Fat if solid at room temperature, oil if liquid at room temperature.
Vegetable oils are 100% triglycerides
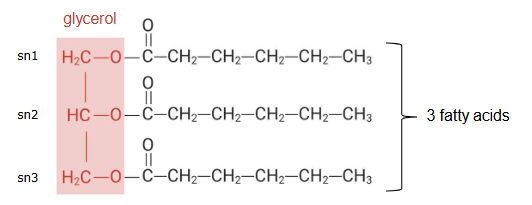
Fatty acids structures
Has a COOH group
They can differ in:
Chain length
Degree of unsaturation
Point of saturation (Where is the double bond located?)
Nomenclature fatty acids
Number of carbons
Number of double bonds
Position of double bonds (for n-designation you count from methyl end + only first double bond, for delta designation you count from carboxyl end and indicates all positions of the double bond)
Major fatty acids in our diet
Palmitic acid (C16:0)
Oleic acid (C18:1, monounsaturated) → most important, takes up about 30 - 40% of fatty acids consumed daily
Linoleic acid (C18:2)
What does fatty acid composition determine?
firmness
stability
solid/liquid at room temperature
The shorter the fatty acids attached, the lower the melting point of the fat.
Solid at room temperature:
More saturated
Less prone to oxidation
e.g. butter
more stable
Liquid at room temperature:
More unsaturated
More prone to oxidation
e.g. sunflower oil
Hydrogenation
conversion of unsaturated → saturated fatty acids
Makes oils more stable and less prone to spoilage
It also leads to the conversion of the cis-fatty acids naturally present in foods into trans-fatty acids.
Trans-fatty acids have a negative impact on health.
Hydrogenation also happens naturally in ruminants → cows and sheep naturally produce trans-fatty acids → present in butter and cheese
Cis vs trans fatty acids
The double bonds within unsaturated fatty acids that are naturally present in foods are in the so called cis configuration → hydrogens on the same side of the double bond.
Gives a kink in the molecular shape.
Trans configuration means that the two hydrogen atoms are bound to opposite sides of the double bond, which results in a more linear fatty acid. These only form in industrial processing of oils.
EXCEPT for conjugated linoleic acid which occurs naturally in dairy.
Digestion of fat
Little fat digestion in mouth (lingual lipase)
Little fat digestion in stomach by gastric lipase
Most takes place in intestine by pancreatic lipase. Happens in the duodenum, later are absorbed by the bile.
Pancreatic lipase breaks down triglycerides into fatty acids in the small intestine.
The monoglycerides form a specific structure called a micelle.
The enterocytes that are found on the villus take up the micelles and monoglycerides.
When they are absorbed they are made back into triglycerides, in a process called re-esterification.
They are transported in little droplets called chylomicrons so that they are soluble in the blood.
The chylomicrons are deposited into the lacteal vessel which is part of the lymphatic system.
Chylomicrons only exist in your blood stream
Chylomicrons structure
Surface coat: unesterified cholesterol, phospholipids, apolipoproteins
Core: cholesteryl esters
Triglycerides
What is the fat used for and how does this happen?
Storage (most end up here)
As a form of energy in the muscles
Fatty acid signaling (in the heart)
How this happens?
Chylomicrons in the bloodstream encounter the enzyme lipoprotein lipase which can be found on the epithelial cells in the blood vessels
Lipoprotein lipase breaks down the triglycerides into fatty acids
These fatty acids are then taken up into the tissues and used for one of the three reasons listed above.
What transports triglycerides?
Chylomicrons
VLDL = very-low-density lipoproteins.
What transports cholesterol?
Low density lipoproteins
High density lipoproteins
Function of adipose tissue
Where excess dietary fat is deposited, storage depot for energy
Heat insulation
Endocrine function (play a major role in regulation of energy metabolism and food intake)
Fate of fat in fasted state
Stored triglycerides are broken down into single fatty acids and released into the bloodstream to be used by the body.
Uses a specific lipase.
Fat fluctuations
Fat release will exceed fat storage at certain times of the day (at night or before meals)
Other times fat storage exceeds fat release (after a meal)
Over the course of 24 hours, fat storage and release will balance out.
ATP
Battery of the body and is “charged” by breaking down fuels found in triglycerides, carbohydrates and proteins. Heat is released
Beta-oxidation
Inside the mitochondria the fatty acyl-CoA undergoes a series of four enzymatic reactions, collectively known as beta-oxidation, which repeat until the fatty acid is completely broken down.
Sterols
Fat soluble molecules
Sterols in foods: cholesterol + plant sterols/stanols (plant version of cholesterol)
The main sterol present in animal foods is cholesterol
Cholesterol is NOT a nutrient
Plants do not contain cholesterol, instead, they contain plant sterols and stanols which resemble cholesterol.
Plant sterols
similar structure
Differ from cholesterol at one branch point
Only tiny amounts of plant sterols are absorbed, most ingested plant sterols leave the body via the stools
They inhibit cholesterol absorption and cholesterol levels in the blood to a maximum of 10%
Cholesterol levels in the blood
<5.0 mmol/l = normal
5.0-6.4 mmol/l = somewhat increased
6.4-7.9 mmol/l = increased
>8.0 mmol/l = strongly increased
Atherosclerosis
Gradual narrowing of (coronary) arteries due to build up of plaque
Occurs in everyone, but initially remains asymptomatic
Obstruction reduces oxygen supply: ischemia
If it happens near the heart it is called ischemic heart disease.
In the brain it may lead to stroke
Outside both of those its peripheral vascular disease
LDL vs HDL
LDL (consists of 2/3 cholesterol) → Deposits cholesterol in the arteries
HDL (consists of 1/3 cholesterol) → Picks cholesterol up
LDL evidence for CHD
Epidemiological
High LDL is correlated with higher CHD risk
Genetic
People with genetically high LDL levels have higher risk for CHD
Pharmacological:
Lowering of LDL using drugs reduces CHD risks
HDL evidence for CHD
Epidemiological
High HDL is correlated with lower CHD risk
Genetic
People with genetically high HDL levels do not have a lower risk for CHD
Pharmacological
Raising HDL using drugs does not reduce CHD risk
Effect trans fats on blood lipids
Trans fat raises blood LDL and lowers HDL when compared with saturated or unsaturated fat
Prospective epidemiological studies indicate that trans fat increases CHD risk
Fat substitution
Mouth feel = similar to fat
Olestra is an example
Sucrose polyester
Not absorbed
Side effect such as steatorrhea (fat induced diarrhea)
Affects absorption of lipid-soluble vitamins
Approved for snacks in the US, not approved in the EU
Constraints of fasting
Does not last indefinitely
Degradation of protein stores (muscle tissue, other organs) for energy should be avoided
Fat largest energy reserve: however, fat can not be converted to glucose
The brains is not able to use fatty acids as energy the brain needs glucose
Energy storage
Protein: as structural and functional protein in muscle and other tissue (~15,000 kcal)
Carbohydrate: as glycogen in liver and muscle (~2500 kcal in total)
Fat: triglycerides in fat tissues (100,000+ kcal)
Brain metabolism
Can not use fatty acids as energy source
low levels of B-oxidation
Protect brain mitochondria from oxidative stress
Glucose primary energy source
Accounts for 60% of glucose used by the body
brain metabolism
Can not use fatty acids as energy source
Low levels of B-oxidation
Protect brain mitochondria from oxidative stress
Glucose primary energy source
Accounts for 60% of glucose used by the body.
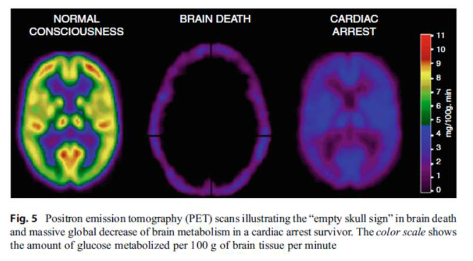
Picture shows brain glucose consumption/utilization in the brain during different situations
Early phase of starvation
no more glucose enters the bloodstream from diet
Consequences: blood glucose levels drop <5mM
Need endogenous source of glucose to maintain blood glucose levels
Endogenous: growing or originating from within an organism
From glycogen stored in liver
Glycogenolysis and gluconeogenesis
Gluconeogenesis: a metabolic pathway that results in the generation of glucose from certain non-carbohydrate carbon substrates
Glycogenolysis: breakdown of glycogen to glucose.
Glucose metabolism during starvation
Once no new food enters the body, the body relies on glycogen supplies in the liver to function. This glycogen will run out after a while (one day max) after which gluconeogenesis will take place.
Early phase of starvation: lipid metabolism
Fat breakdown (lipolysis) in adipose tissue becomes activated
Consequence: plasma free fatty acid and glycerol levels increase
Plasma free fatty acids and glycerol levels continue to rise
Fatty acids are taken up by the liver
more fatty acids enter the liver than can be fully oxidized
Excess fatty acids are converted to ketone bodies or triglycerides
Fasting leads to ketogenesis and fatty liver.
Importance of ketone bodies
Body needs an alternative fuel for the brain to replace glucose
Ideally, this would be fatty acids as fat is abundant, but these can not be used by brain
Instead, the brain can use an intermediate product of fatty acid oxidation: ketone bodies
However, ketone bodies can only be synthesized in the liver and this process takes time.
Use of resources from fed to fasting to starvation
Fed:
The body will first use readily available nutrients
Fasting:
The body will use glucose from glycogen
Then free fatty acids from adipose tissue
Then ketone bodies from free fatty acids
Starvation:
Glucogenesis
Distinguish between the different lipoproteins
Chylomicrons:
Transport dietary triglycerides from the intestine to tissues
Lowest density
VLDL:
Transports triglycerides from the liver to tissues.
Low density
LDL:
Delivers cholesterol to cells
Medium density
HDL:
Removes excess cholesterol from the bloodstream and tissues
Highest density
Lowest density = largest molecule