chemistry term2/3
5.0(1)
5.0(1)
Card Sorting
1/65
Earn XP
Description and Tags
Study Analytics
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
66 Terms
1
New cards
periodic table
an ordered arrangement of all 118 known elements. The elements are arranged in order of their atomic number (number of protons). Every time you move an element to the right, the proton number increases by 1
2
New cards
predicting chemical properties
all elements in a column have the same number of electrons in their outer shell, they have similar chemical properties. This means they will all react in similar ways.
3
New cards
groups
the name of columns of a periodic table. The number of the column is equal to the amount of electrons on the outer shell.
\
\
4
New cards
periods
the name of rows of a periodic table. The table is ‘periodic’ because elements with similar properties occur at regular intervals, i.e. periodically. Therefore, the rows of the periodic table are called periods.
\
\
5
New cards
John Newland
the first chemist to devise a periodic table. His periodic table was ordered by the weight of the element. However, the table was incomplete, and some elements were placed in the wrong groups.
6
New cards
Dmitri Mendeleev
\
The man who created the modern day periodic table. He realised that there may be undiscovered elements. He added gaps to Newlands’ table to account for undiscovered elements. He even predicted the properties and masses of these undiscovered elements!
\
The man who created the modern day periodic table. He realised that there may be undiscovered elements. He added gaps to Newlands’ table to account for undiscovered elements. He even predicted the properties and masses of these undiscovered elements!
\
7
New cards
metals
They are found on the left of the periodic table because they have few electrons in their outer shell. When they react, they lose 1 or more of these negatively charged electrons to form positively charged ions. They also commonly react with oxygen to form oxides
8
New cards
Properties of metals
.High melting and boiling points
.good conductors of heat and electricity.
.They are all solids at room temperature apart from mercury.
.good conductors of heat and electricity.
.They are all solids at room temperature apart from mercury.
9
New cards
Non-metals
They are found on the right of the periodic table because they have many electrons in their outer shell. When they react, they either Gain electrons to form negatively charged ions or share electrons to form neutral molecules (covalent bonding)
10
New cards
properties of non-metals
.lower melting point
.low boiling point
\
.low boiling point
\
11
New cards
electronic structure
Electrons fill an atom's shells in order of increasing energy. The closer a shell is to the nucleus, the lower its energy level, so the first shell that is filled is the closest to the nucleus.
12
New cards
electron shells
Electrons have fixed positions in atoms called xxx. xxx go around the atom's nucleus.
13
New cards
electron configuration
A way tells us how an atom's electrons are organised.
.The inner-shell (closest to the nucleus) can have a maximum of 2 electrons and the next two shells can have a maximum of 8 electrons. Any extra electrons are then put into a next shell.
.The inner-shell (closest to the nucleus) can have a maximum of 2 electrons and the next two shells can have a maximum of 8 electrons. Any extra electrons are then put into a next shell.
14
New cards
key feature of a chemical reaction
.An energy change that can be measured
.At least one new substance is created
.If compounds are broken up or formed.
.At least one new substance is created
.If compounds are broken up or formed.
15
New cards
Key features of a physical change
.No new substance is formed.
.Reversible change
Chemical property of substance remains the same
.Change in colour, shape, size, and state
\
.Reversible change
Chemical property of substance remains the same
.Change in colour, shape, size, and state
\
16
New cards
alkali metals
.group 1 metals
.malleable
.can conduct heat and electricity
.highly reactive
.low density and melting points
\
.malleable
.can conduct heat and electricity
.highly reactive
.low density and melting points
\
17
New cards
why group1 metals are highly reactive
They are highly reactive because they give away their electron faster to form a positive charge.
18
New cards
transition metals
compared to alkali metals, they have:
.higher melting points
.higher density
.lower reactivity
.higher melting points
.higher density
.lower reactivity
19
New cards
properties of transition metals
.can form ions with different positive charges.
.are often used as catalysts as they easily lend and take electrons from other electrons.
.can form colourful compounds.
.are often used as catalysts as they easily lend and take electrons from other electrons.
.can form colourful compounds.
20
New cards
types of chemical bonds
atoms can for 3 types of bonds, ionic bonding, covalent bonding or metallic bonding.
21
New cards
ionic bonding
a bonding which involves an attraction between oppositely charged ions. These bonds are found in compounds made of metals and non-metals.
22
New cards
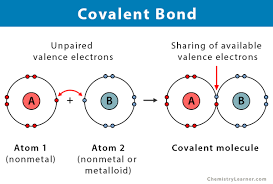
covalent bonding
a bonding which involves 2 non-metal atoms sharing 1 or more pairs of electrons. These bonds are found in most non-metal elements and in compounds of non-metals. The bond is so strong because the electrons are attracted to the nucleus
23
New cards
metallic bonding
a bonding which involves an attraction between positively charged ions and negatively charged, delocalised electrons. These bonds are found in metals and alloys (mixtures of metals and other substances).
24
New cards
delocalised atom
atoms on the outer shell that are free to move
25
New cards
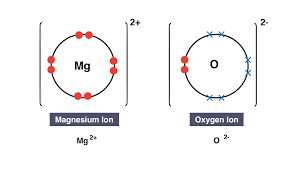
dots and cross diagrams
a diagrams that shows electrons being transferred and ions being formed. Dots represent electrons from 1 atom and crosses represent electrons from the other atom. Square brackets and a charge (e.g. 2+) represent ions
26
New cards
cations
atoms with less electrons than protons
27
New cards
anions
negative ions. atoms with more electrons than protons
28
New cards
noble gases
positive ion. Group 0 elements. They already have a full outer shell. They are unreactive and don't normally form ionic bonds with other elements.
29
New cards
properties of ionic compounds
\
.only conducts electricity when molten or dissolved (cannot conduct as a solid)
.high melting points
.only conducts electricity when molten or dissolved (cannot conduct as a solid)
.high melting points
30
New cards
identifying ionic compounds
An ionic compound formula has no overall charge.
31
New cards
naming ionic compounds
\
.Ionic compounds made from 2 different elements end in -ide.
.Ionic compounds made from 3 or more different elements end in -ate.
\
.Ionic compounds made from 2 different elements end in -ide.
.Ionic compounds made from 3 or more different elements end in -ate.
\
32
New cards
ionic compounds
ionic compounds form when millions of metal atoms transfer their outer electrons to millions of non-metals at the same time. The resulting oppositely charged ions are held together in ionic lattices
33
New cards
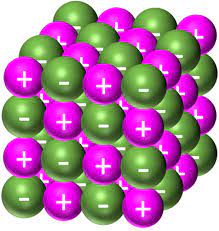
ionic lattices
ionic lattices are giant structures ( a regular repeating arrangement) that are held together electrostatic forces between positive and negative ions.
34
New cards
valence electrons
What you call electrons that are on the outermost shell.
35
New cards
empirical formulae
the simplest ratio of ions possible. eg 1calcium 2 bromides is CaBr₂
36
New cards
covalently bonded substances (examples)
water, polyester, diamond
37
New cards
Intramolecular bonds
forces between molecules; covalent bonds. They are strong
38
New cards
intermolecular forces
the force that holds together small molecules. They are weak.
39
New cards
small covalent molecules
Small molecules bonded together by intramolecular bonds called covalent bonds and held together by weak intermolecular forces.But they are weak and easy to break which means they have low boiling and melting point. They are often liquids and gases at room temperature.
40
New cards
Intermolecular Forces and Molecule Structure
The size of a molecule affects the overall strength of intermolecular forces. The strength of intermolecular forces affects the properties of a molecule.
41
New cards
properties of small covalent structure
\
It is usually gas or liquid at room temperature.
It has a low boiling and melting point
weak intermolecular bonds
they don’t conduct electricity because they have no delocalised electron.
It is usually gas or liquid at room temperature.
It has a low boiling and melting point
weak intermolecular bonds
they don’t conduct electricity because they have no delocalised electron.
42
New cards
properties of giant covalent structures
.They have very high melting and boiling points
.They have no specific empirical formula.
.No intermolecular force because they exist as 1 large molecule
.They have no specific empirical formula.
.No intermolecular force because they exist as 1 large molecule
43
New cards
polymers
.large, chain-like molecules made up of repeating units that can extend for thousands of atoms. They are held together by Strong covalent bonds between atoms in molecules and Weak intermolecular forces between molecules. Because of the large size of polymer molecules, the intermolecular forces add up to be quite strong.
44
New cards
Properties of polymers
.They are usually solid when at room temperature
.They melt easily as the intermolecular forces remain less strong than chemical bonds but add up to be quite strong due to the large size.
.They melt easily as the intermolecular forces remain less strong than chemical bonds but add up to be quite strong due to the large size.
45
New cards
addition polymerisation
a process in which monomers, which are small organic molecules, are chemically bonded together to form a polymer, which is a long chain of repeating units.
46
New cards
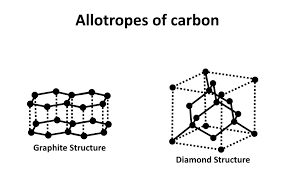
diamond
A giant covalent structure which is an allotrope (form) of carbon.
47
New cards
allotrope
The existence of a chemical element in two or more forms
48
New cards
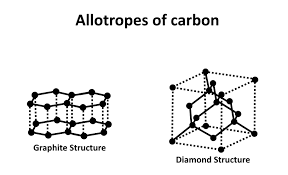
properties of diamond
.High melting point
.It is hard-lots of strong covalent bonds
.does not conduct electricity because there are no delocalised electrons in the structure.
\
.It is hard-lots of strong covalent bonds
.does not conduct electricity because there are no delocalised electrons in the structure.
\
49
New cards
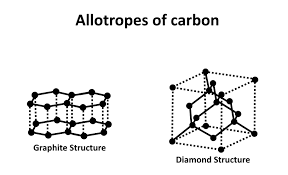
Graphite
Graphite is an allotrope (form) of carbon.
50
New cards
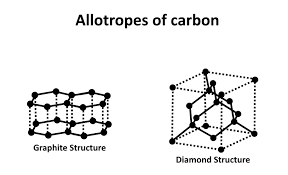
properties of graphite
.it is soft-form layers of hexagonal (6-sided) rings, with weak intermolecular forces keeping the layers together. The layers can easily slide over one another
.conducts electricity
.conducts electricity
51
New cards
uses of graphite
52
New cards
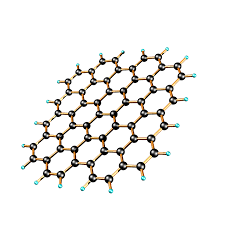
graphene
graphene is an allotrope form of carbon.
53
New cards
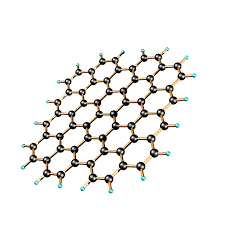
properties of graphene
.It conducts electricity
.It is light but strong (a single layer of graphite)
.It is light but strong (a single layer of graphite)
54
New cards
uses of graphene
Its used in electronics (batteries, solar panels, phone screens). It makes them stronger while not making them heavier.
55
New cards
fullerenes
Fullerenes are molecules of carbon atoms that take up hollow structures. Their structure is usually carbon atoms arranged in hexagonal (6-sided) rings, but pentagonal (5-sided) and heptagonal (7-sided) carbon rings can also be found.
56
New cards
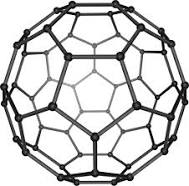
Buckminsterfullerene
A spherical fullerene. It was the first to fullerene that was discovered and its formula is C₆₀. It is technically a simple molecule because of its fixed size.
57
New cards
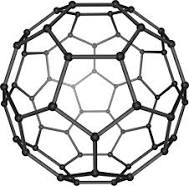
Uses of Buckministerfullerenes
It can be used as Catalysts, Lubricants ,As vehicles for transporting drugs into our bodies.
58
New cards
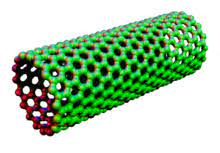
cylindrical fullerenes
Carbon nanotubes which are often called molecular wires because they have a tiny diameter but can be incredibly long.
59
New cards
Uses of cylindrical fullerenes
The strength and electrical conductivity of nanotubes make them useful In electronics, in nanotechnology and For strengthening materials (e.g. tennis racket frames).
60
New cards
metallic bonding
Metallic bonds are the electrostatic attraction between positive ions and negative delocalised electrons. The structure is a regular lattice of positive ions (cations) in a ‘sea’ of delocalised electrons.
61
New cards
delocalised electrons
Delocalised electrons are not bound to an atom and are free to move around within the lattice. Delocalisation happens because metal atoms have a small number of electrons in their outer shells.
62
New cards
pure metals
Metals that have giant structures with strong electrostatic forces between positive ions and delocalised electrons. All of the ions are the same size and these ions are arranged in layers.
63
New cards
properties of pure metals
.High melting and boiling points
.soft and malleable because ions are arranged in layers
.soft and malleable because ions are arranged in layers
64
New cards
alloys
A combination of 2+ elements, where at least 1 is a metal.The ions of the different elements are different sizes.
\
\
65
New cards
Why alloys are harder than pure metals
The ions are different sizes, distorting the regular layer, making it harder for layers to slide unlike pure metals which is why they’re in construction.
66
New cards
why metals are great conductors of heat and electricity
Metals conduct heat and electricity well. This is because the delocalised electrons that are around the layers of positive ions can carry a charge through the structure. The electrons move from the negative terminal to the positive terminal.