Unit 1.1 - Chemical Elements are joined together to form biological compounds
1/85
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
86 Terms
What are Inorganic Ions? - Key Terms
All organisms need inorganic ions to survive; these inorganic ions are often called minerals.
Micronutrients are minerals (inorganic ions) needed in minute (trace) concentrations e.g. copper and zinc.
Macronutrients are inorganic ions needed in small concentrations e.g. magnesium and iron.
What are Organic + Inorganic Molecules?
Organic – Molecules that have a high proportion of carbon and hydrogen atoms.
Inorganic – A molecule or ion that has no more than one carbon atom.
What are the Symbols + Biological Roles of the following Inorganic Ions? Magnesium, Iron + Calcium
Magnesium - Mg2+ Constituent of chlorophyll and therefore essential for photosynthesis
Iron - Fe2+ Constituent of haemoglobin, which transports oxygen in red blood cells
Calcium - Ca2+ Hardens bones and teeth (not strengthen). Also a component of plant cell walls.
What are the Symbols + Biological Roles of the following Inorganic Ions? Nitrate + Phosphate
Nitrate - NO3- Nitrogen derived from nitrate is needed for making nucleotides, including ATP, DNA and RNA. Nitrogen is also needed for amino acid formation.
Phosphate - (PO4)3- Used for making nucleotides, including ATP, DNA and RNA. A constituent of phospholipids found in biological membranes. Hardens bones.
What is Sulfur used for?
Sulfur is required to synthesise some amino acids / methionine / cysteine / proteins.
What is Water? - Key Terms
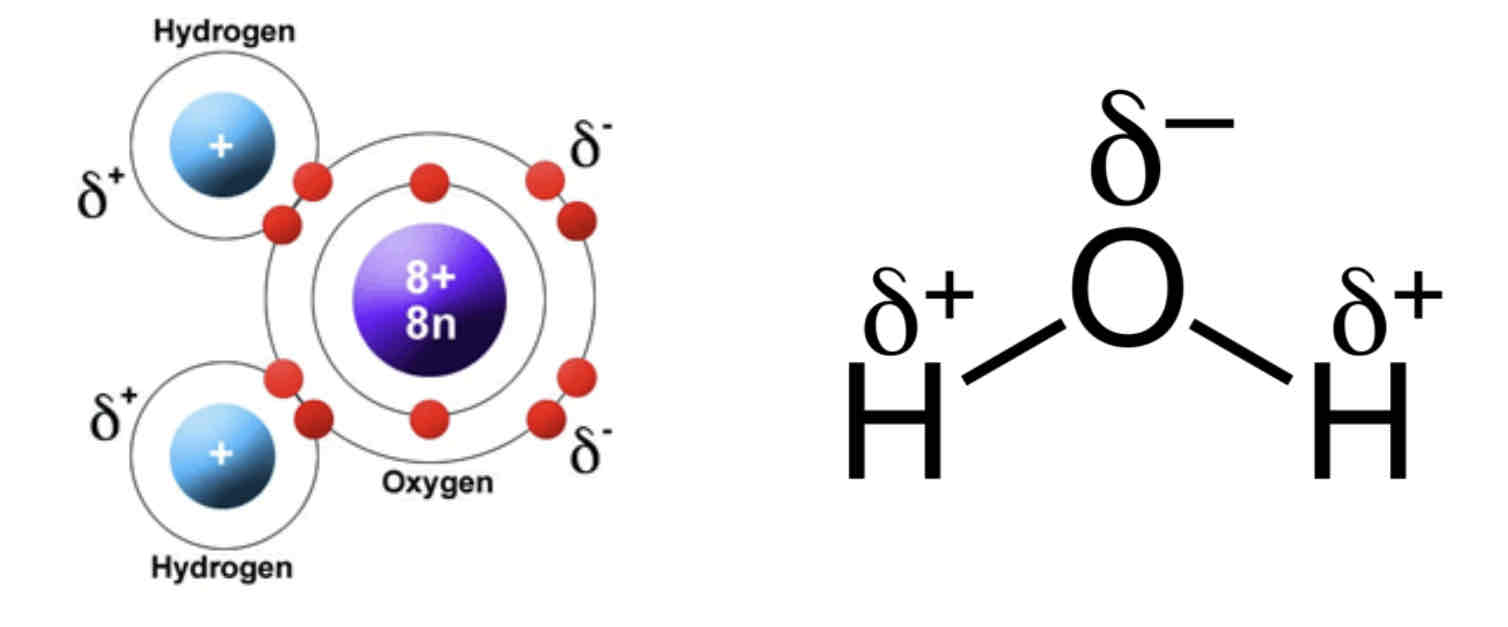
Water has the chemical formula of H20. Water consists of one oxygen atom joined to two hydrogen atoms by covalent bonds.
Water is a polar molecule; the oxygen end of the molecule has a negative charge and the hydrogen atoms have a positive charge. This uneven distribution of charge is called a dipole.
Dipole – A polar molecule which has a positive and negative charge, separated by a very small distance.
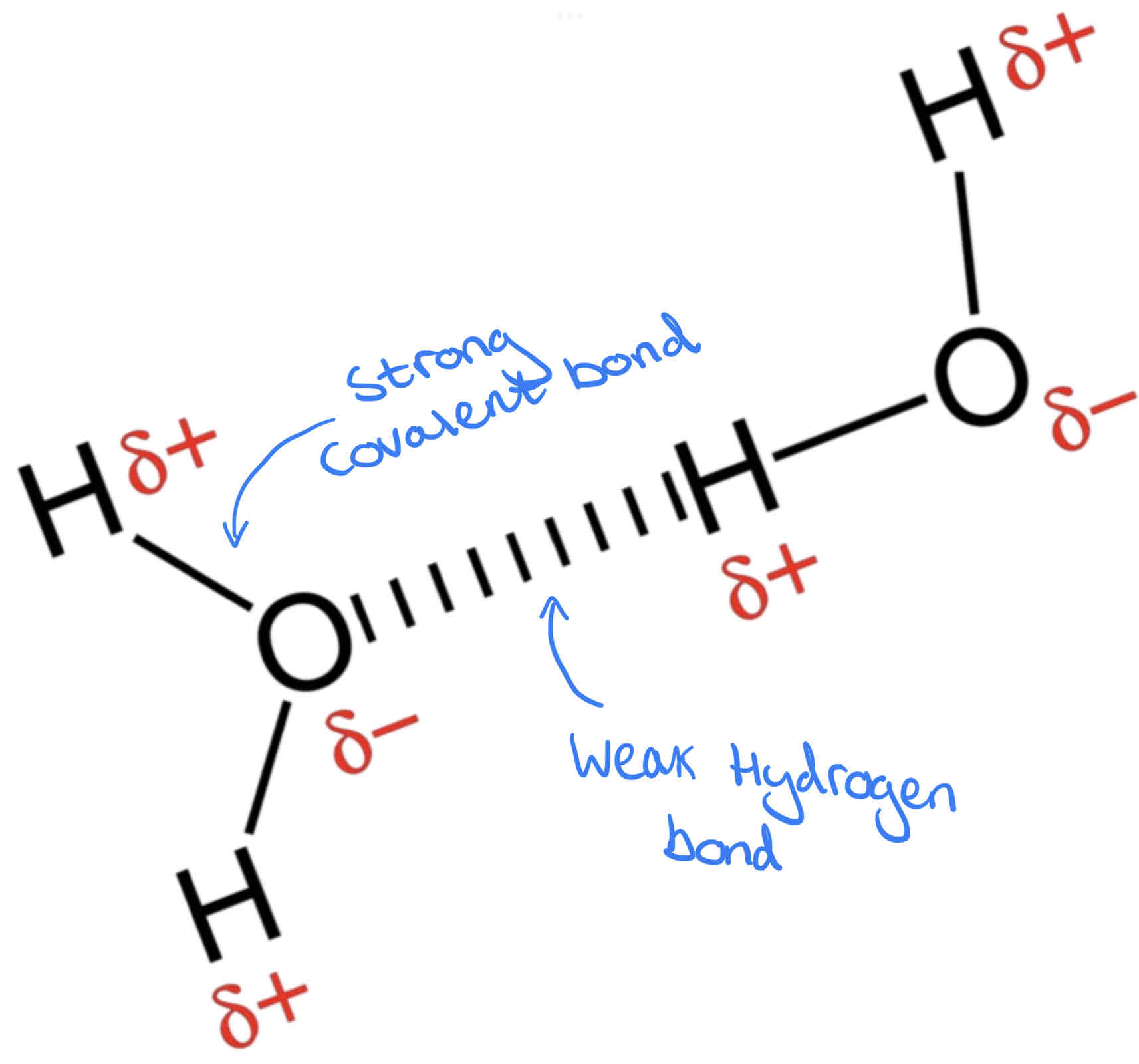
What happens when 2 Water Molecules join together?
When two water molecules are in close contact the opposing charges attract each other forming a hydrogen bond. Individually hydrogen bonds are weak, but many hydrogen bonds (between many water molecules) form a lattice-like framework which is much stronger. This attraction between water molecules is called cohesion.
What is a Hydrogen Bond?
Hydrogen bond – The weak attractive force between a hydrogen atom (with a partial positive charge) and an atom with a partial negative charge, usually oxygen or nitrogen.
What are the Properties of Water?
Water is a solvent
Water as a transport medium
Chemical reactions take place in water
Water has a high specific heat capacity
Water has a high latent heat of vaporisation
Cohesion
Surface tension
Density
Colourless
Properties of Water - Water is a Solvent - Function
The positive and negative parts of the water molecule attract other charged particles, such as ions and other polar molecules, such as glucose.
Ions and polar molecules can dissolve in water. Non-polar molecules such as lipids do not dissolve in water.
Minerals, Oxygen and Carbon Dioxide can dissolve in water.
Polar molecules and ions can dissolve and therefore be transported.
Function of Water as a transport medium
Blood is largely water and transports many dissolved substances around the body. Minerals dissolved in water are transported from the root to the leaves via the xylem in plants.
Why do Chemical reactions take place in water?
Water is a Metabolite.
All chemical reactions occur in aqueous solutions. Polar molecules and ions can dissolve and therefore be transported.
Transport of ions and polar molecules allows chemical reactions to take place when particles or molecules meet.
Why does Water have a high specific heat capacity?
A large amount of heat energy is needed to raise the temperature of water. This prevents large fluctuations in water temperature.
This keeps the temperature of aquatic environments stable so that organisms do not have to endure extremes of temperature. This also allows enzymes within cells to work effectively.
Why does Water have a high latent heat of vaporisation?
Due to cohesion between water molecules (caused by hydrogen bonding) a large amount of heat energy is needed to change water from a liquid to a vapour state (gas). This process of evaporation transfers heat energy and is a very effective way of cooling the body e.g. sweating or panting. Evaporation of water from a surface causes cooling.
What is the Function of Cohesion as a Property of Water?
The attraction between water molecules, caused by hydrogen bonding allows water to be transported, in long columns, up the xylem vessels of even the tallest trees.
Surface tension in Water - Function
At ordinary temperatures water has the highest surface tension of any liquid except mercury. In a pond the cohesion between water molecules (caused by hydrogen bonding) supports organisms, such as pond skaters, allowing them to walk on water.
Function of Density as a Property of Water
Water has a maximum density at 4 oC; ice is less dense and therefore floats on the surface and insulates the water beneath it. This reduces the tendency for large bodies of water to freeze completely allowing organisms to survive.
Why is Water Colourless/ Transparent?
Colourless with a high transmission - Light can pass through cells for Photosynthesis.
Minerals can be absorbed for Photosynthesis.
What are the 3 Macronutrients?
Carbohydrates
Lipids
Proteins
What are Macronutrients? + Examples
Macronutrients are organic compounds. This means they contain a carbon chain joined to atoms of hydrogen & oxygen
(& sometimes nitrogen, sulphur & phosphorus).

Why is Carbon Important?
Binds strongly to other C atoms.
Can make 4 covalent bonds.
Small molecules (monomers) can join together to form large molecules (polymers).
Can form branched chains & rings
What is a Carbohydrate?
Carbohydrates are organic compounds which contain the atoms carbon, hydrogen and oxygen. The basic unit of a carbohydrate is a monosaccharide. Two monosaccharides form a disaccharide. Many monosaccharide molecules form a polysaccharide. A polysaccharide is a type of polymer.
Carbohydrates - Key Facts
Includes sugars, starch, glycogen & cellulose.
Can be broken down into usable energy or stored in cells.
What are the 3 Main groups of Carbohydrates?
The 3 main groups are: Sugars ending in ‘ose’. Have sweet taste.
Monosaccharides – single (simple) sugars.
Disaccharides – double sugars.
Polysaccharides – multiple sugars.
What are Monosaccharides?
Monosaccharides are sweet and soluble. They are the building blocks for the other larger carbohydrates.
Monosaccharides have the general formula (CH2O)n and they can be grouped according to the number of carbon atoms they have. A triose sugar has three carbon atoms, a pentose sugar has five carbon atoms and a hexose sugar has six carbon atoms.
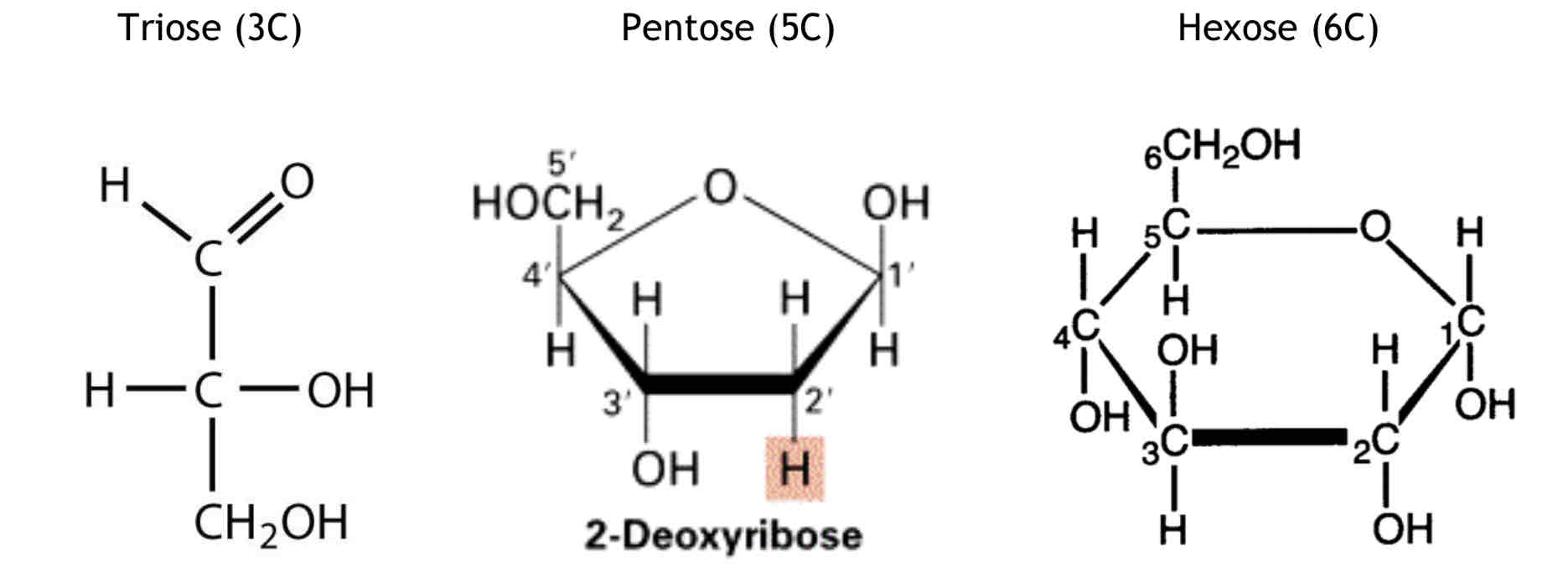
What are the functions of these Monosaccharides?

Carbohydrates - Isomers
Isomers have the same chemical/ molecular formula and the same number of atoms; the atoms are simply arranged differently (different structural formula)
The ring form of the monosaccharide glucose has two isomers α glucose and β glucose.
They both have the same chemical formula C6H12O6, but the H and OH atoms are arranged differently at carbon 1.
What are Disaccharides?
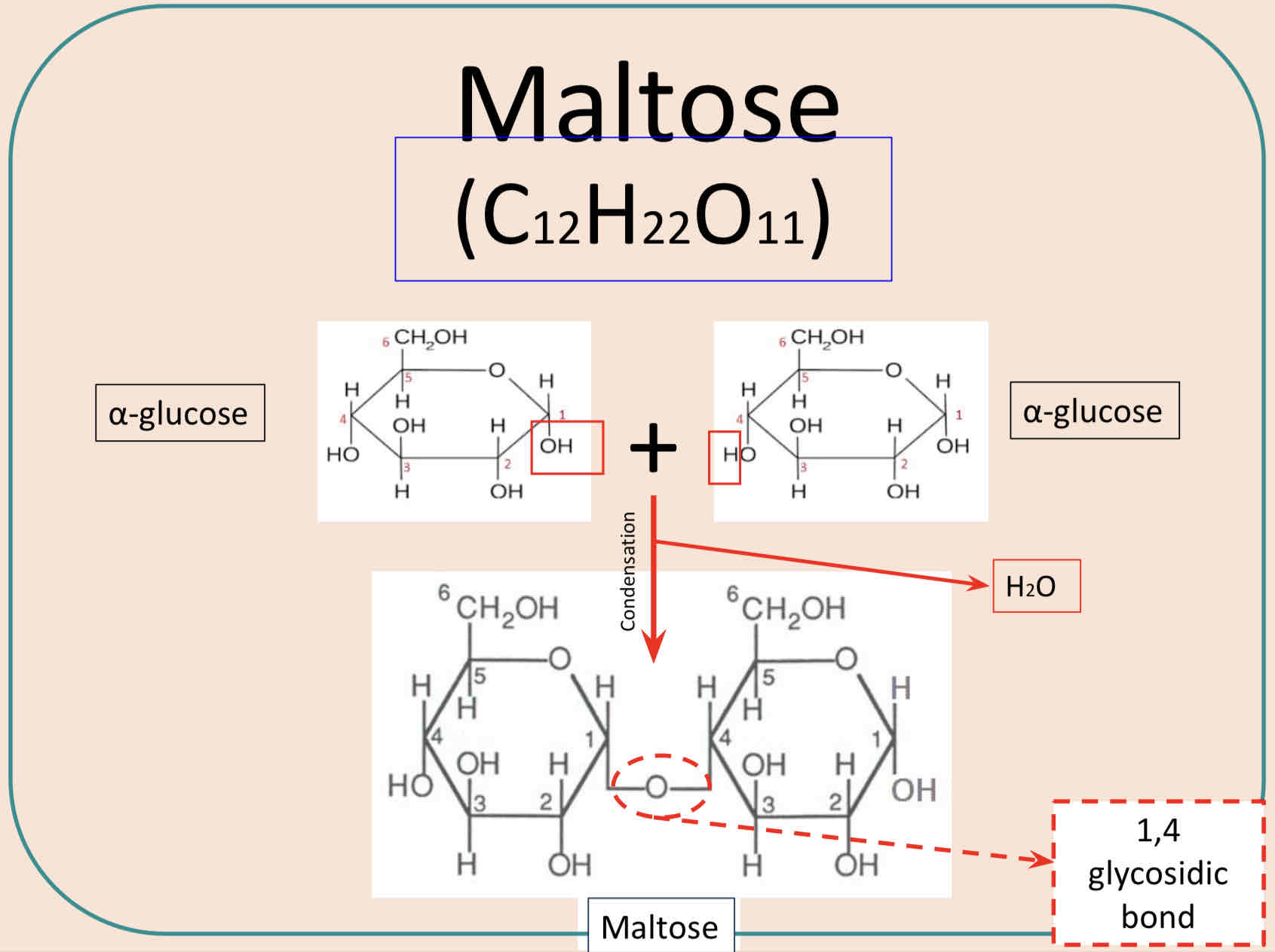
Disaccharides are composed of two monosaccharide sub-units bonded with the formation of a glycosidic bond and the elimination of water. This is an example of a condensation reaction.
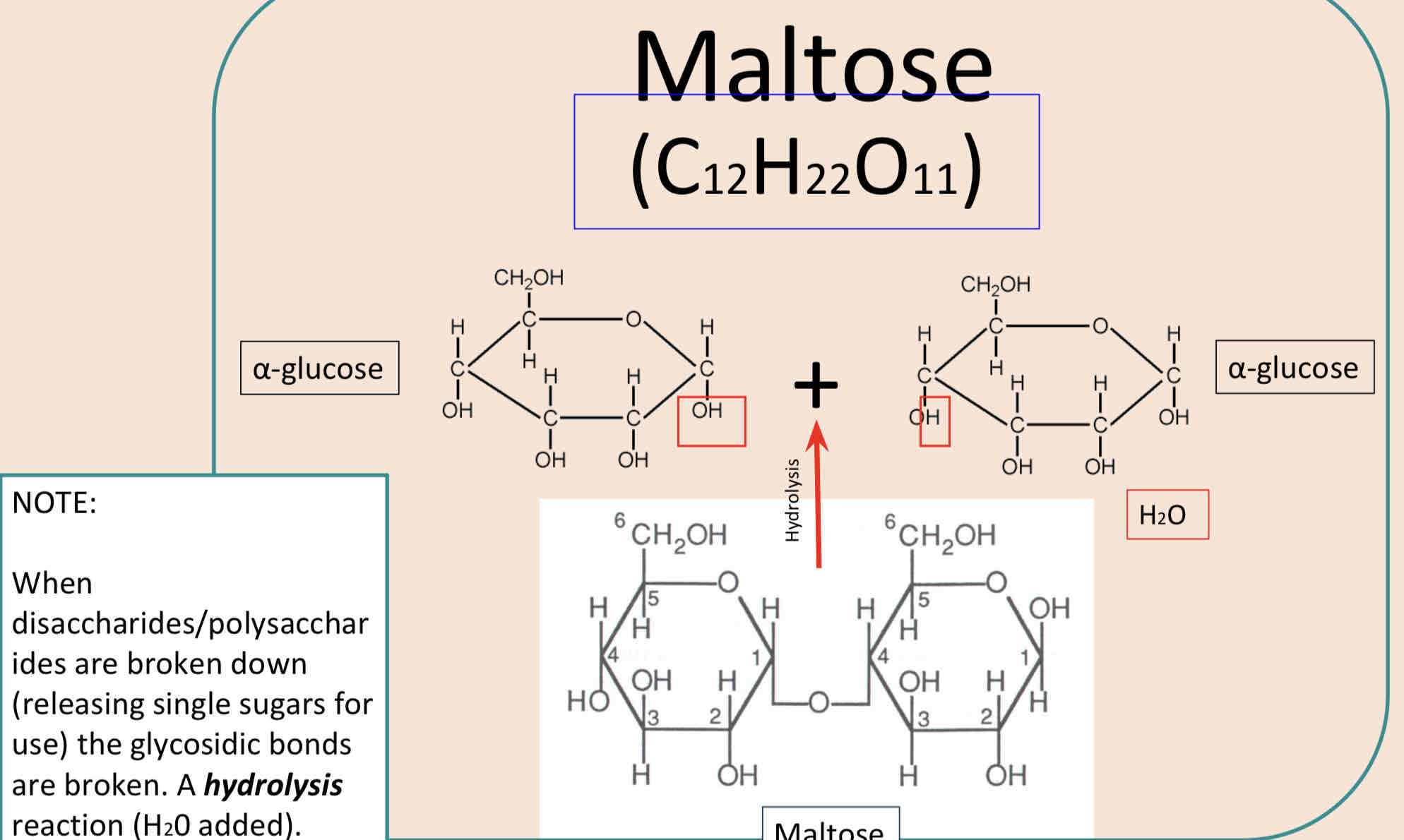
Formation of Maltose
When two α glucose molecules are joined by condensation reaction the disaccharide maltose is formed. The diagram below shows water being removed between C1 of the first glucose molecule and C4 of the second (the atoms removed are shown in red).
A 1-4 glycosidic bond is formed.
The difference between α glucose and β glucose is that the H and OH atoms at C1 are flipped.
Water is released as a byproduct during condensation.
How can the glycosidic bond be broken?
If water is added to the disaccharide, under appropriate reaction conditions, the bond will break, OH ̄ and Hᵻ are added to the C1 and C4 of the broken bond and the 2 α glucose molecules will reform.
This is called a hydrolysis reaction – water used to split a molecule – and is the reverse of the condensation reaction.
The glycosidic bond can be broken by hydrolysis. During hydrolysis water is chemically added to break the glycosidic bond. Hydrolysis of maltose is shown below. The atoms added during hydrolysis are shown in red.
What are the functions of these Disaccharides? Maltose, Sucrose + Lactose
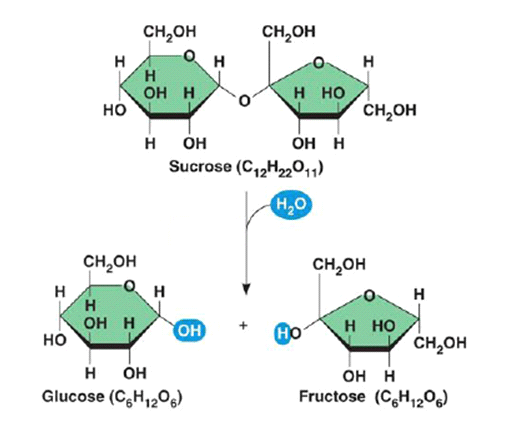
Sucrose Structure
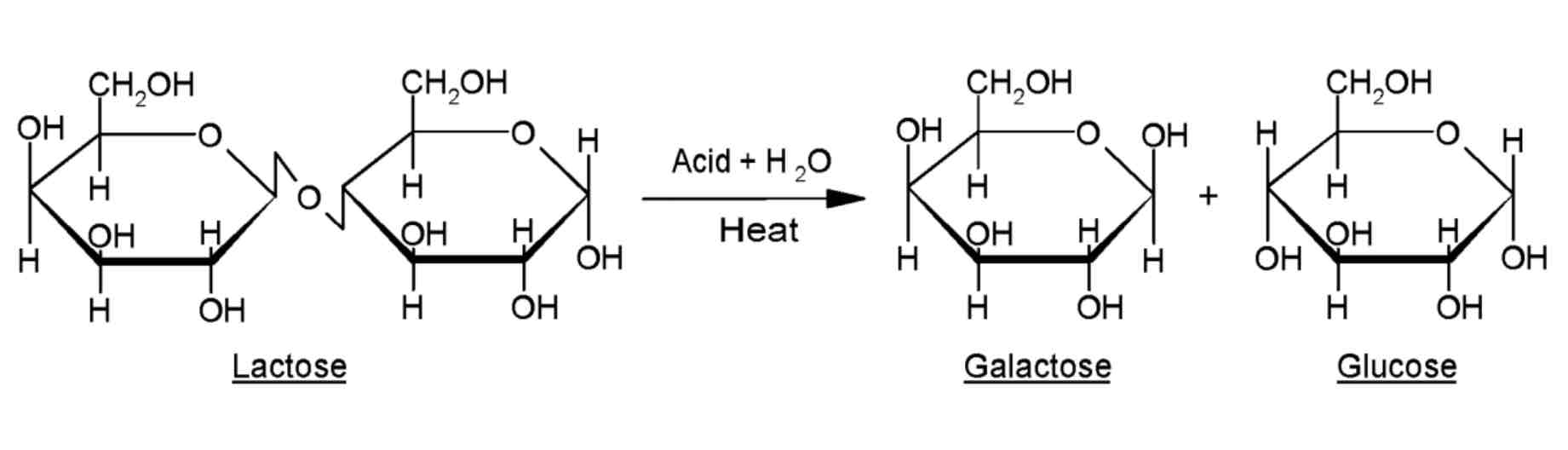
Lactose Structure
The reaction below shows the disaccharide lactose being hydrolysed. The glycosidic bond is broken and the monosaccharides glucose and galactose are formed.
The alternate monosaccharide is flipped so that the OH groups are parallel and can react with one another.
Carbohydrates - What are Polysaccharides?
Polysaccharides are large complex polymers. They are formed from very large numbers of identical monosaccharide units, which are their monomers, linked by glycosidic bonds formed by condensation reaction.
What does Starch allow plants to do? - Storage Polysaccharides
Starch allows plants to store glucose. Starch is a polymer made up of α glucose monomers, added one at a time by condensation reaction. Glucose can be easily added or removed. Starch has two types of polysaccharide, amylose and amylopectin.
Amylose is unbranched and coils; each α glucose monomer added forms a C1 – C4 glycosidic bond with the adjacent glucose molecule.
Amylopectin is branched as it forms C1 - C4 gylcosidic bonds and C1 – C6 glycosidic bonds.
Starch is compact due to branched structure and has no osmotic effect on the cell; it does not affect the water potential of the cell.
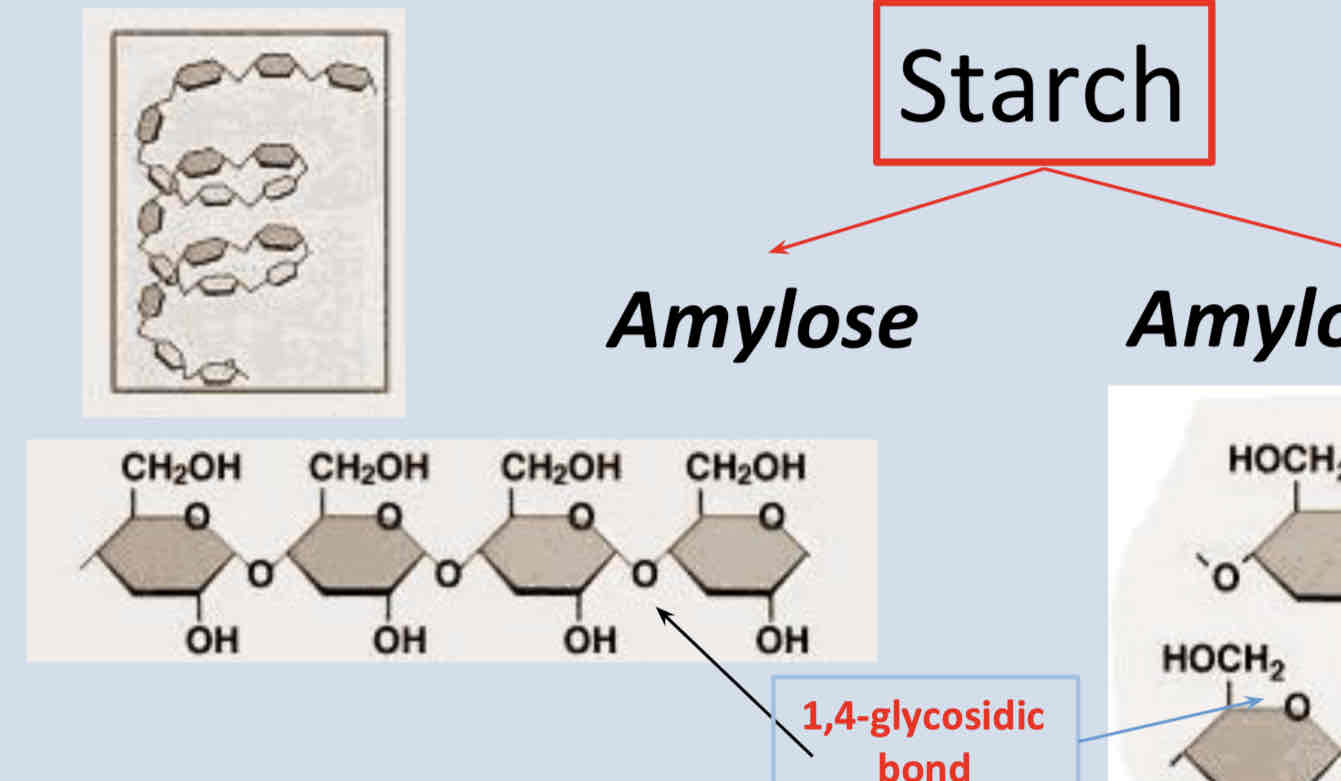
What is Amylose?
Amylose is a polysaccharide component of starch. It is unbranched and coiled
Unbranched polymer
Glucose monomers joint by 1,4-glycosidic bonds.
helical structure - spiral (more compact)
200-5000 glucose monomers.
Glucose released by enzyme action at both ends of polymer (slower)
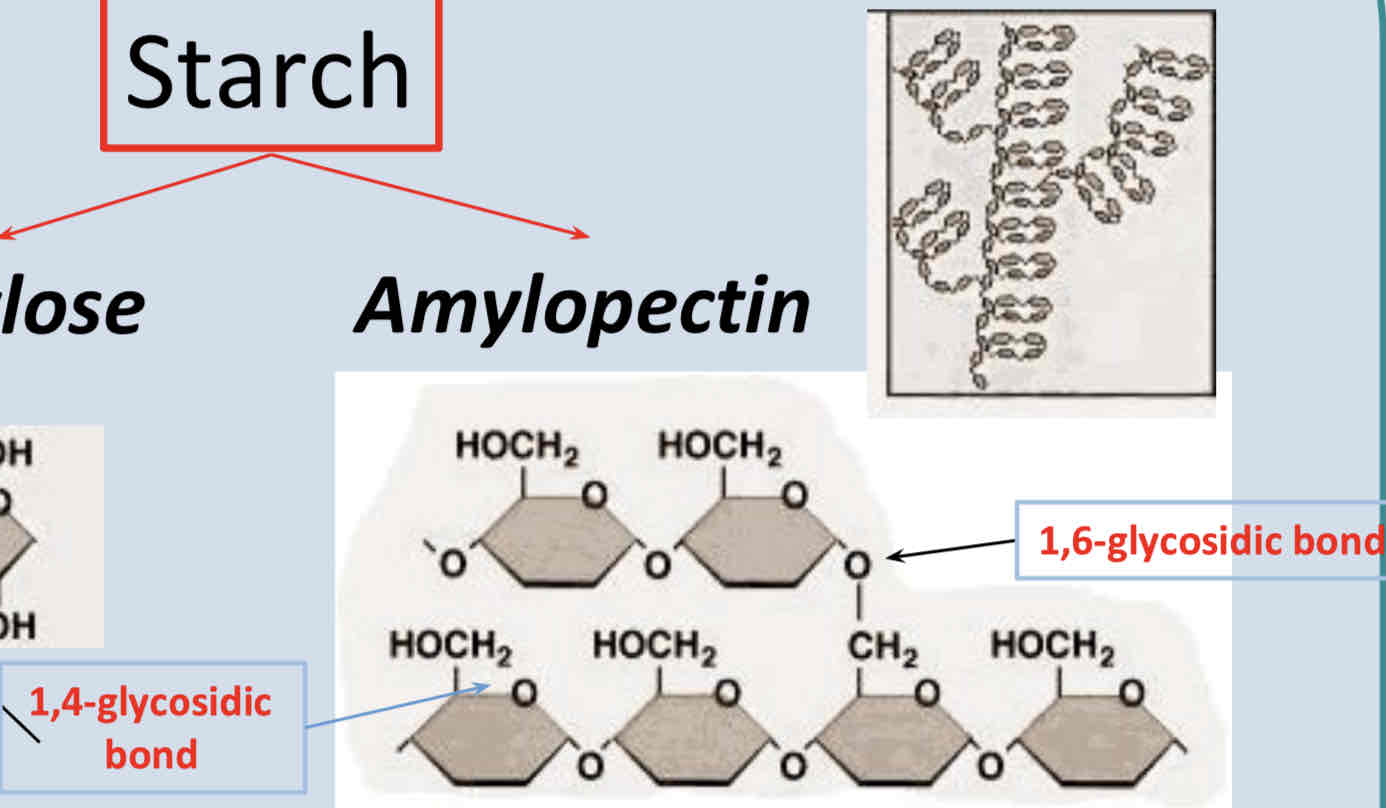
What is Amylopectin?
Amylopectin is a polysaccharide component of starch
Branched polymer
Glucose monomers joint by 1,4-glycosidic bonds & 1,6-glycosidic bonds
Glucose released by enzyme action at all of the endings therefore glucose released rapidly
Describe the role of the three enzymes involved in the complete hydrolysis of Starch [3]
{Starch/ amylopectin/ amylose} is digested to maltose by amylase (1)
Maltose is digested to glucose by {maltase/ an enzyme} (1)
Isomaltase {hydrolyses/ breaks} the amylopectin at the {branch points/ 1-6 glycosidic bonds} (1)
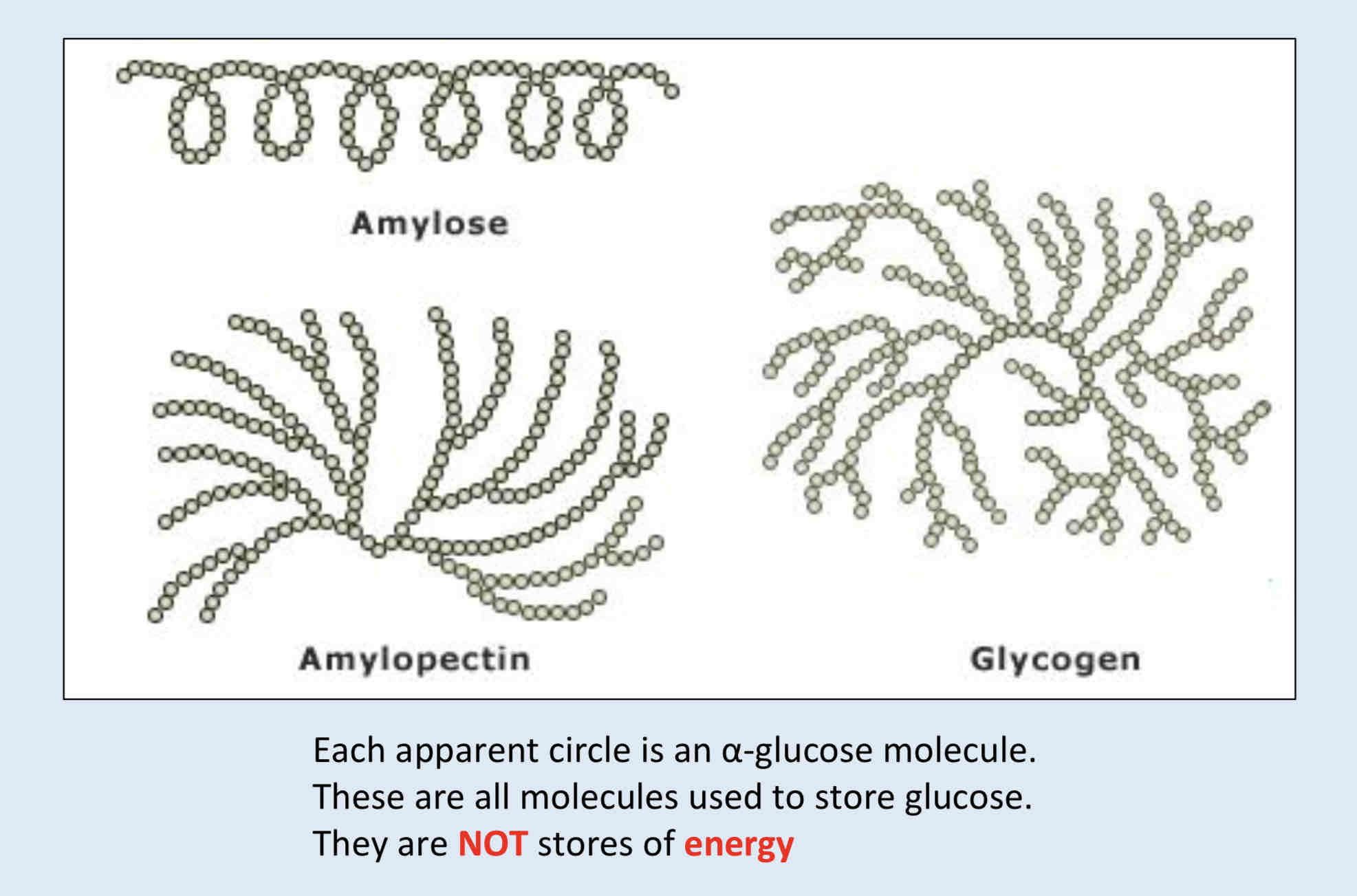
What is Glycogen? + Note
Glycogen is the main storage product in animals. It is similar in structure to amylopectin. In glycogen the α glucose molecules are joined by C1 – C4 and C1 – C6 glycosidic bonds.
The main difference between amylopectin and glycogen is that glycogen has shorter C1 – C4 α glucose chains and there are more C1 – C6 branch points. Glycogen is more branched than amylopectin.
Both starch and glycogen are easily hydrolysed to α glucose, which is soluble and can be transported to wherever energy is needed.
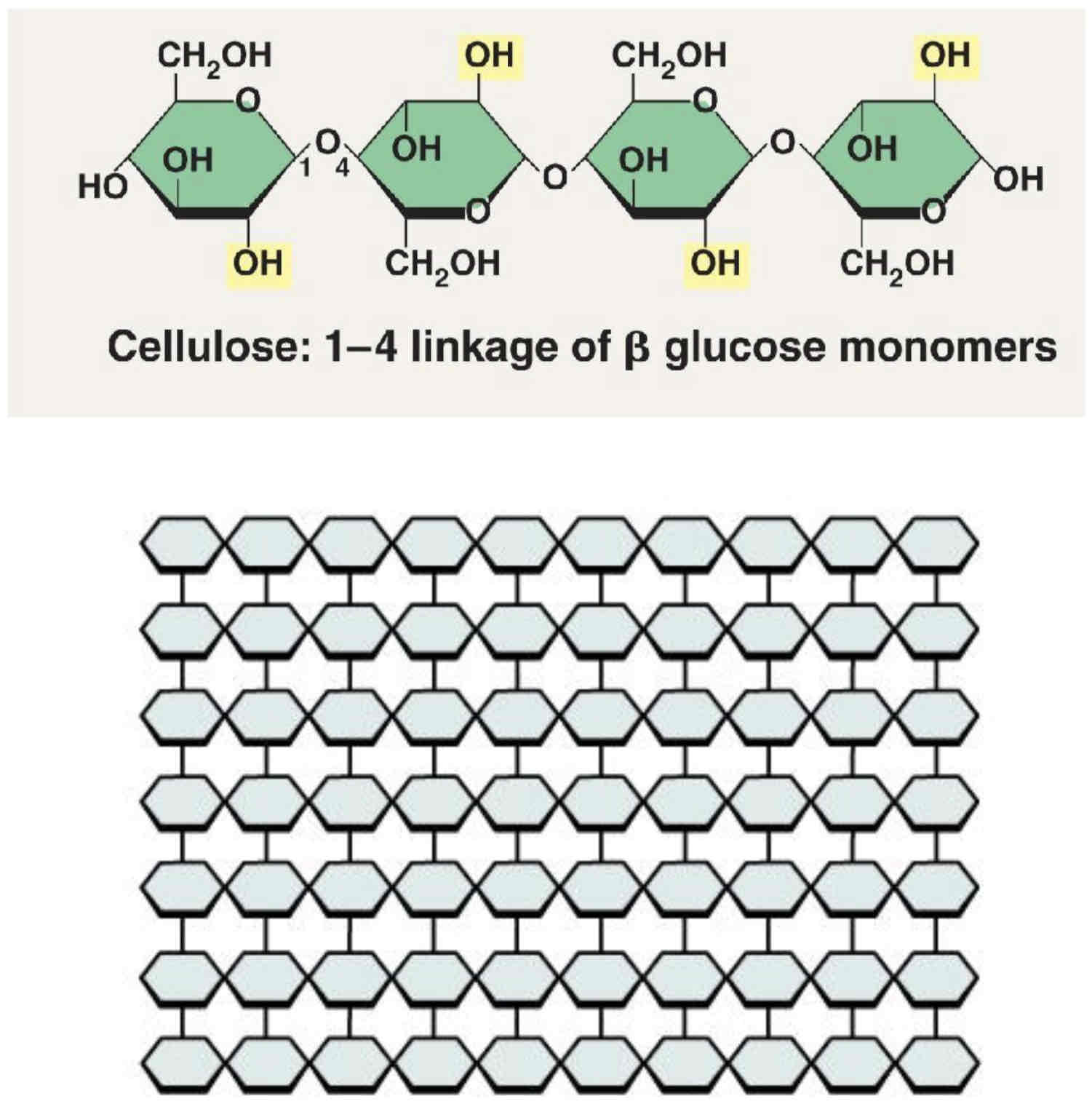
What is Cellulose?
Cellulose is a structural polysaccharide found in plant cell walls. Cellulose consists of many long, parallel chains of β glucose units. The β glucose monomers are joined by C1 – C4 glycosidic bonds. The β bond rotates adjacent glucose molecules by 180o; this allows hydrogen bonds to form between OH groups of adjacent cellulose chains.
Between 60 and 70 cellulose molecules become tightly cross-linked by hydrogen bonds to form bundles called microfibrils. Microfibrils are bunched together in bundles to form fibres.
Cellulose is unreactive and stable (due to being unbranched) and has a high tensile strength (due to the formation of microfibrils and fibres).
What is Chitin?
Chitin has a similar structure to cellulose. It is a structural polysaccharide found in the exoskeleton of arthropods, such as insects, and fungal cell walls. Chitin is composed of long chains of β glucose molecules linked by C1 – C4 glycosidic bonds.
Chitin differs from cellulose in that each monomer has a group derived from amino acids added, called an acetylamine group. Like cellulose alternate glucose molecules are rotated by 180o; this allows hydrogen bonds to form between the OH groups of adjacent chitin chains.
The cross-linked parallel chains caused by hydrogen bonds form microfibrils. Chitin is strong, waterproof and lightweight.
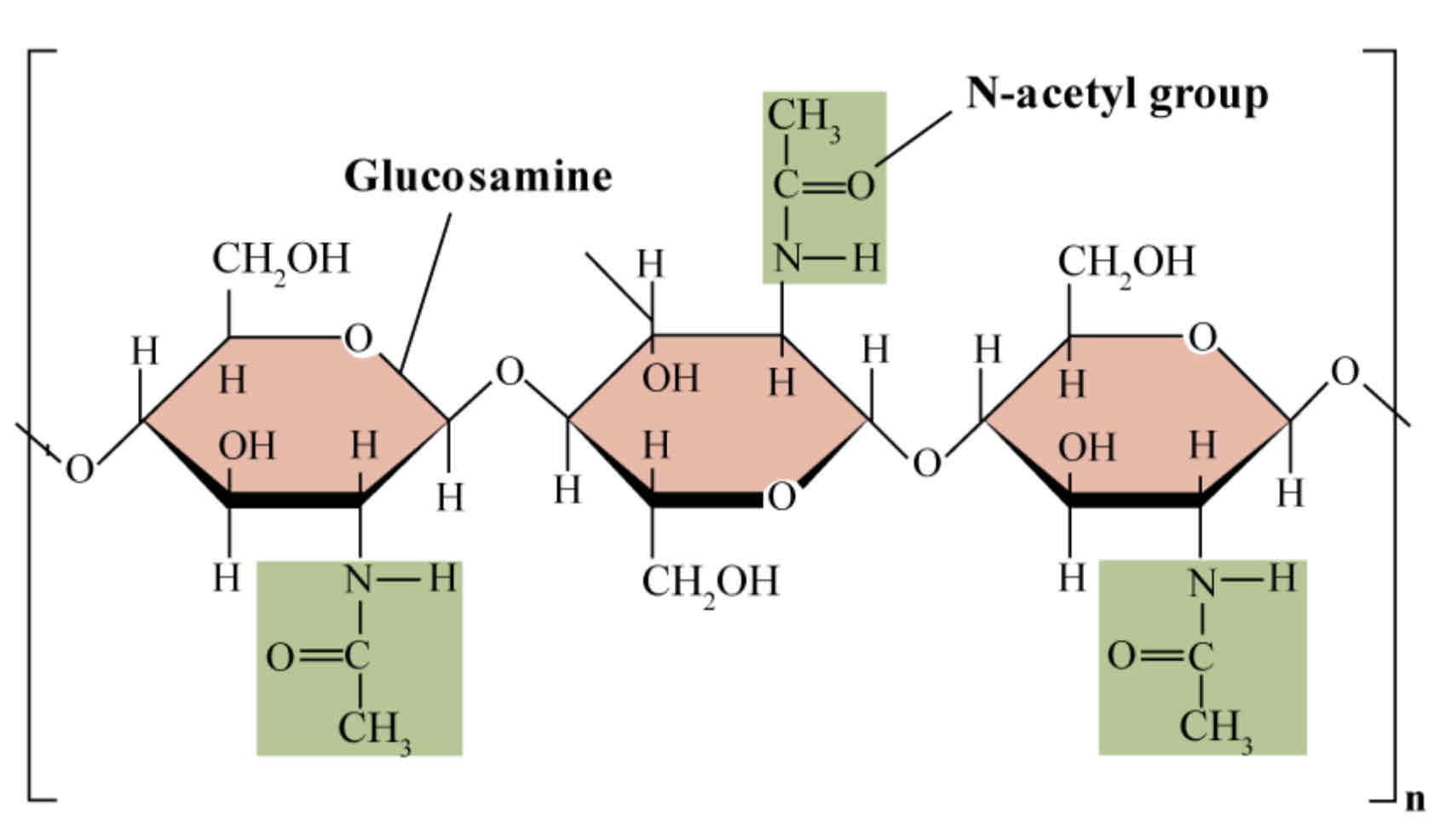
Key Terms - Polymer, Condensation Reaction + Hydrolysis
Key Terms:
Polymer – A large molecule comprising of repeated, identical units (monomers) bonded together.
Condensation reaction – Water is chemically removed to form a bond between adjacent monomers.
Hydrolysis – Water is chemically added to break a bond between monomers.
Proteins - Amino Acids
Proteins differ from carbohydrates and lipids in that, in addition to carbon, hydrogen and oxygen atoms, they also always contain nitrogen atoms. Many proteins also contain sulphur and phosphorus atoms too.
Proteins are polymers made of monomers called amino acids. A chain of amino acids is called a polypeptide. There are 20 different amino acids.
There are thousands of different proteins and their shape is determined by the specific sequence of amino acids in the chain. The shape of a protein determines its function.
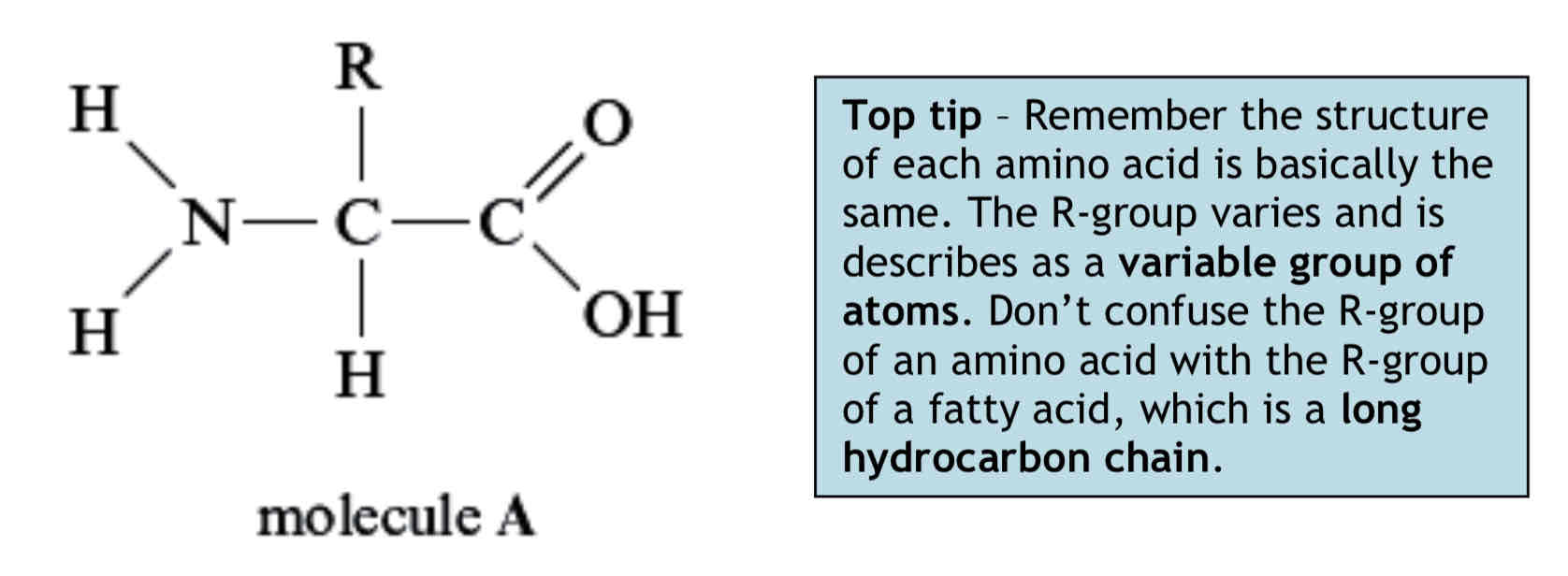
Structure of Proteins - Amino Acids
All amino acids have the same basic structure. Attached to a central carbon atom are:
An amino group (-NH2), which is basic or alkaline.
A carboxyl group (-COOH), which is acidic.
A hydrogen atom.
The R-group, which is a variable group of atoms.
There are 20 different R-groups. The simplest amino acid is glycine which has a hydrogen atom as its R-group. The basic structure of an amino acid is shown below (molecule A):
Are Amino Acids essential?
Amino acids can be essential or non-essential. Essential amino acids cannot be synthesised by our bodies, and must be provided by our diet. Non-essential amino acids can be synthesised by our bodies.
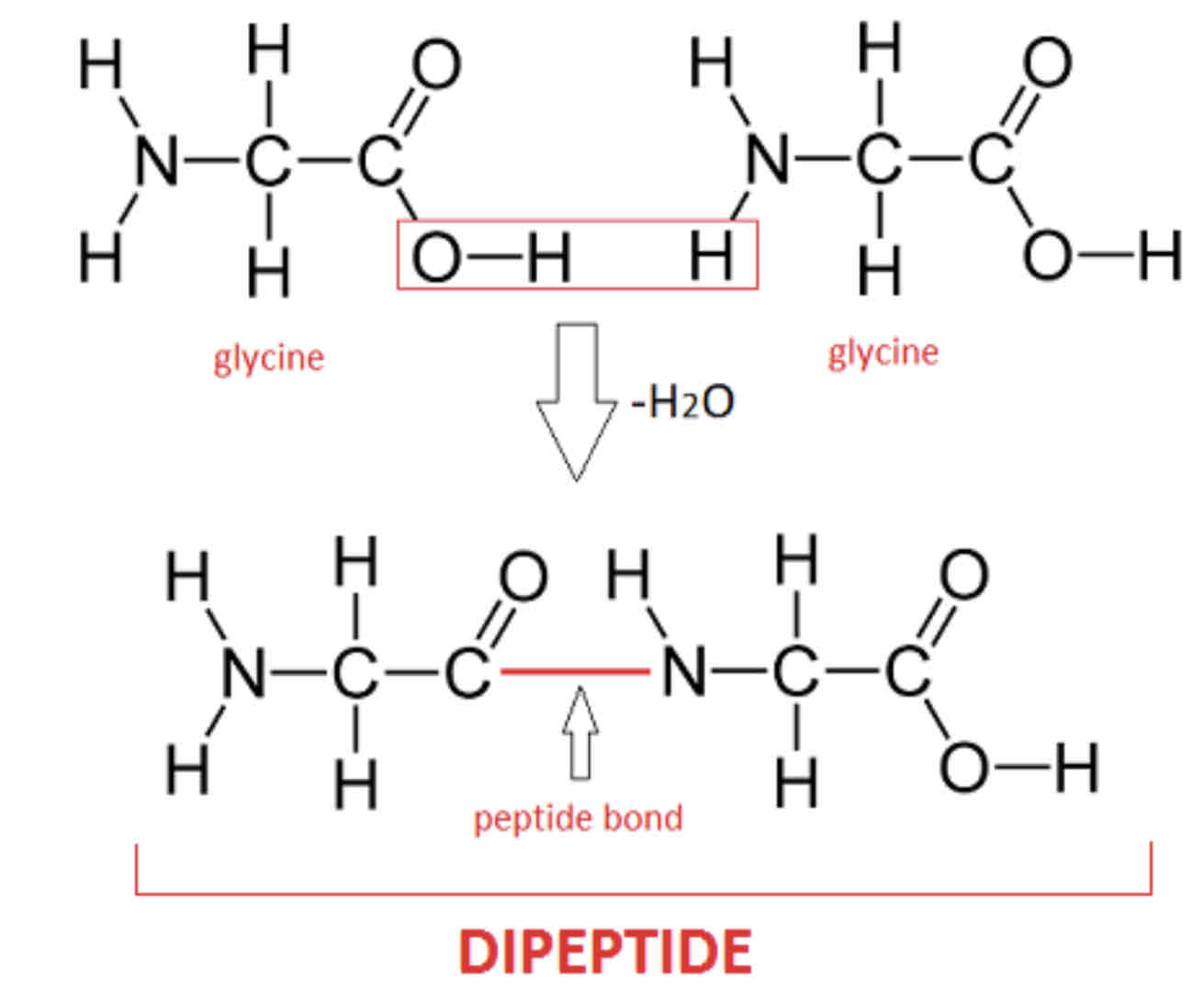
Proteins – Dipeptides and polypeptides
Proteins are linear sequences of amino acids. The amino group of one amino acid reacts with the carboxyl group of another by condensation reaction; water is eliminated and a peptide bond is formed. The resulting compound is a dipeptide.
More amino acids can be added in this way to form a polypeptide molecule which is a type of polymer. The polypeptides can be further modified to form protein molecules each with specific structures and functions.
Water is produced as a byproduct.
Four levels of Protein Structure - Primary
The primary structure is the sequence of amino acids in a polypeptide chain joined with peptide bonds formed during condensation reaction.
The sequence of amino acids is determined by DNA; one gene codes for one polypeptide.
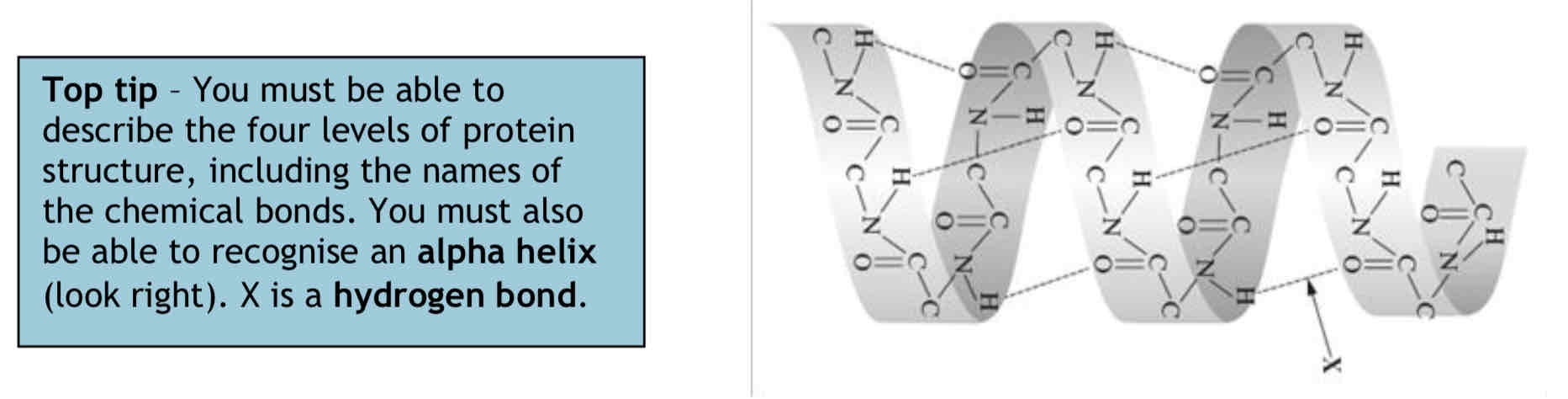
Four levels of Protein Structure - Secondary
The secondary structure is the shape that the polypeptide chain forms due to hydrogen bonding. Hydrogen bonds twist and fold the polypeptide forming an alpha helix or a less common beta pleated sheet.
Four levels of Protein Structure - Tertiary
The alpha helix of a secondary protein structure is further folded and twisted to give a more complex, compact 3D structure.
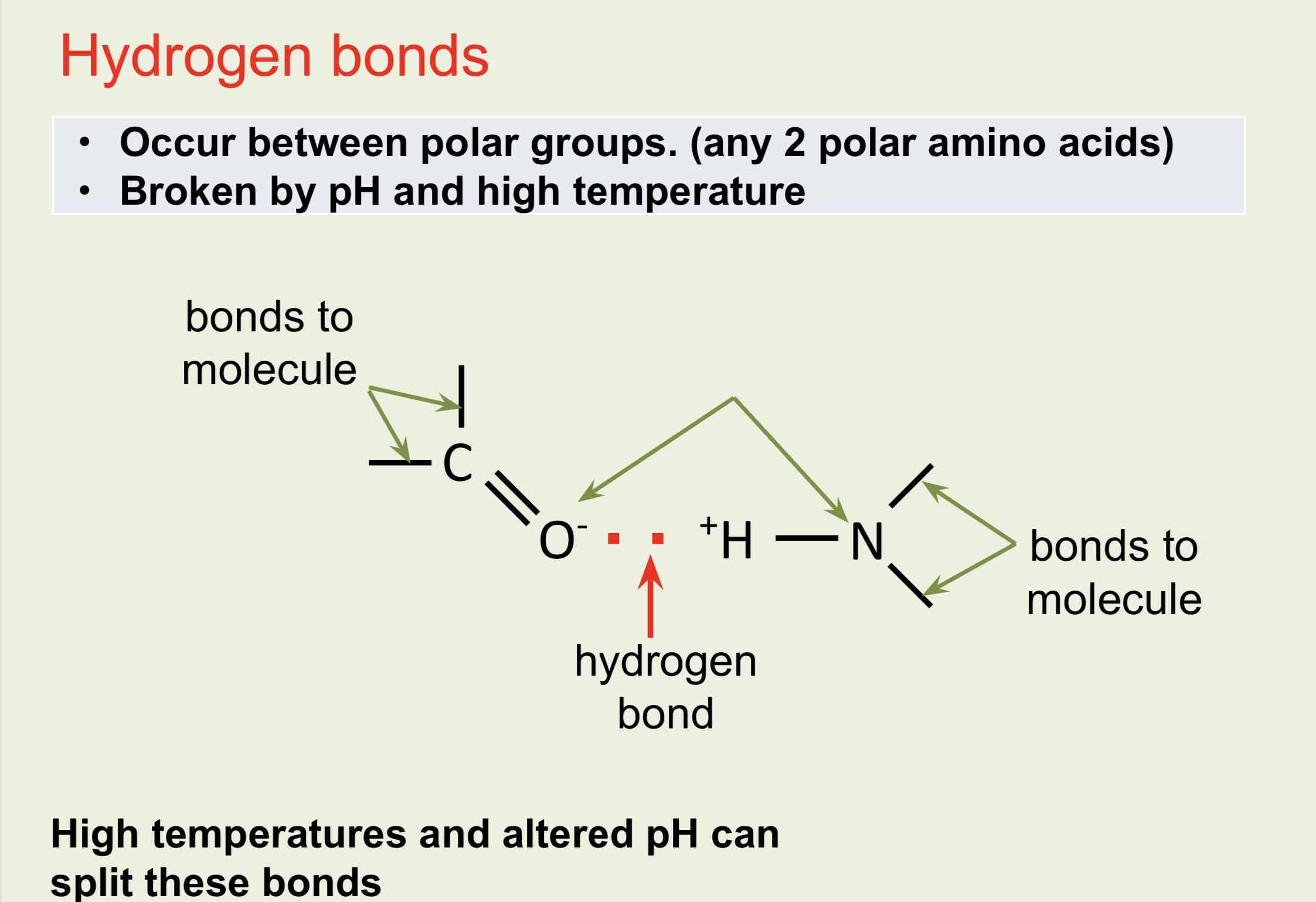
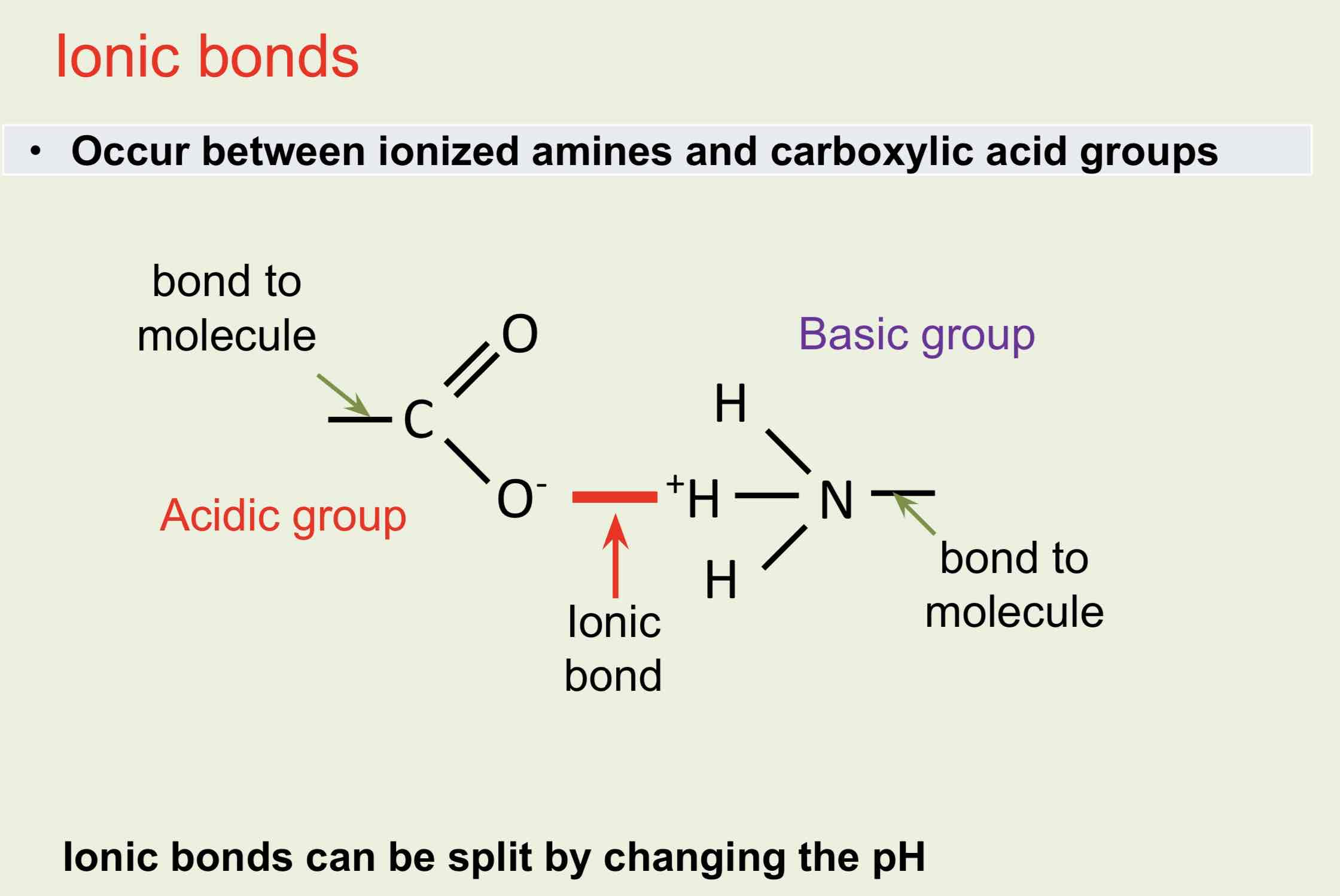
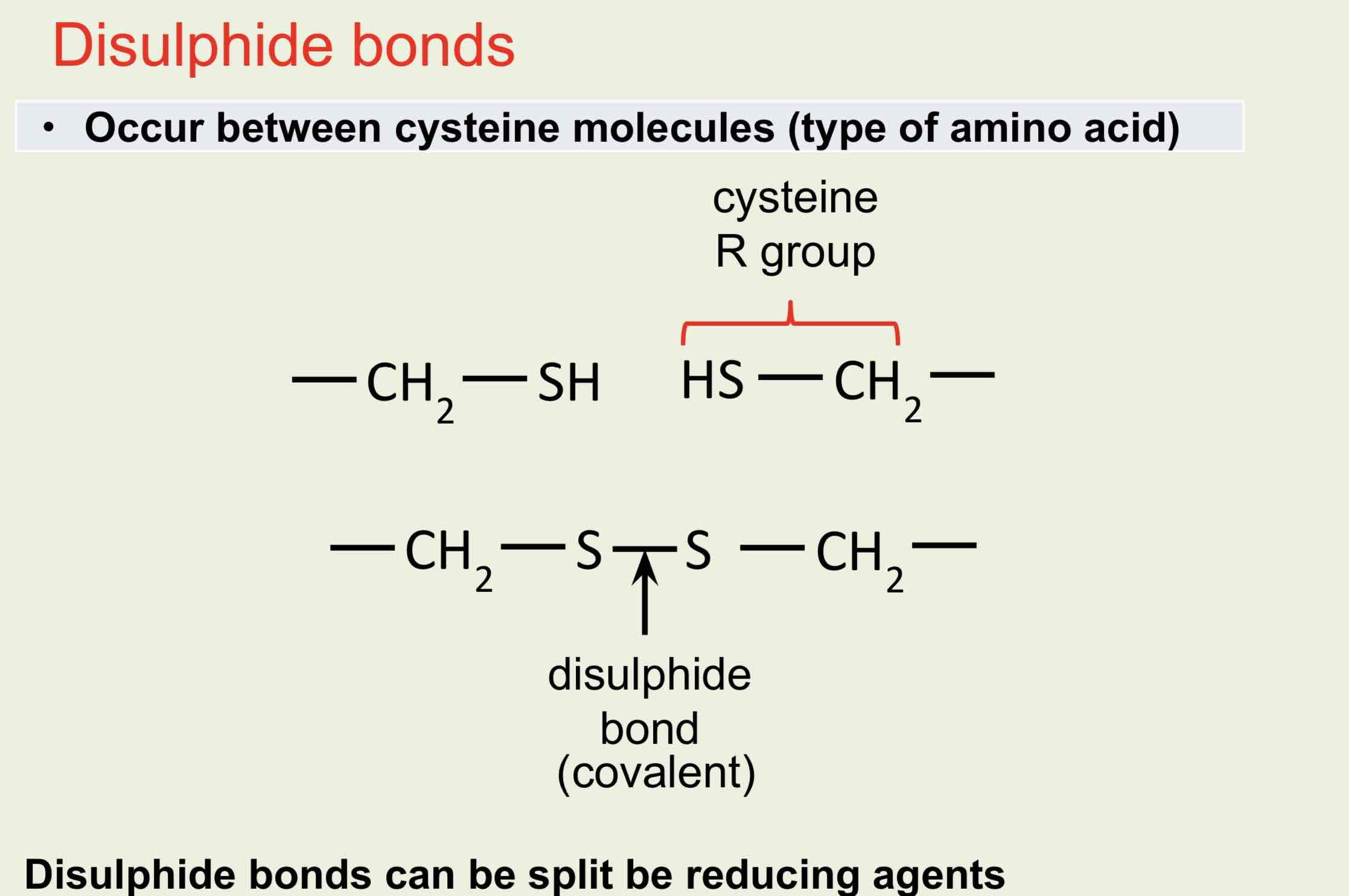
The shape is maintained by disulphide, ionic, covalent hydrophobic (van der Waal’s forces) and hydrogen bonds between R-groups.
Enzymes have a tertiary protein structure. The bonds maintain the shape of the enzyme’s active site.
Four levels of Protein Structure - Quaternary
The quaternary structure arises from a combination of two or more polypeptide chains in tertiary form.
These are associated with non-protein groups and form large complex molecules such as haemoglobin.
Haemoglobin has four polypeptide chains.
Four genes are needed to code for haemoglobin; one gene for each polypeptide.
Define Quarternary Structure
Two or more polypeptide chains {bonded/ joined} together (to form a functional protein).
Protein Bonds
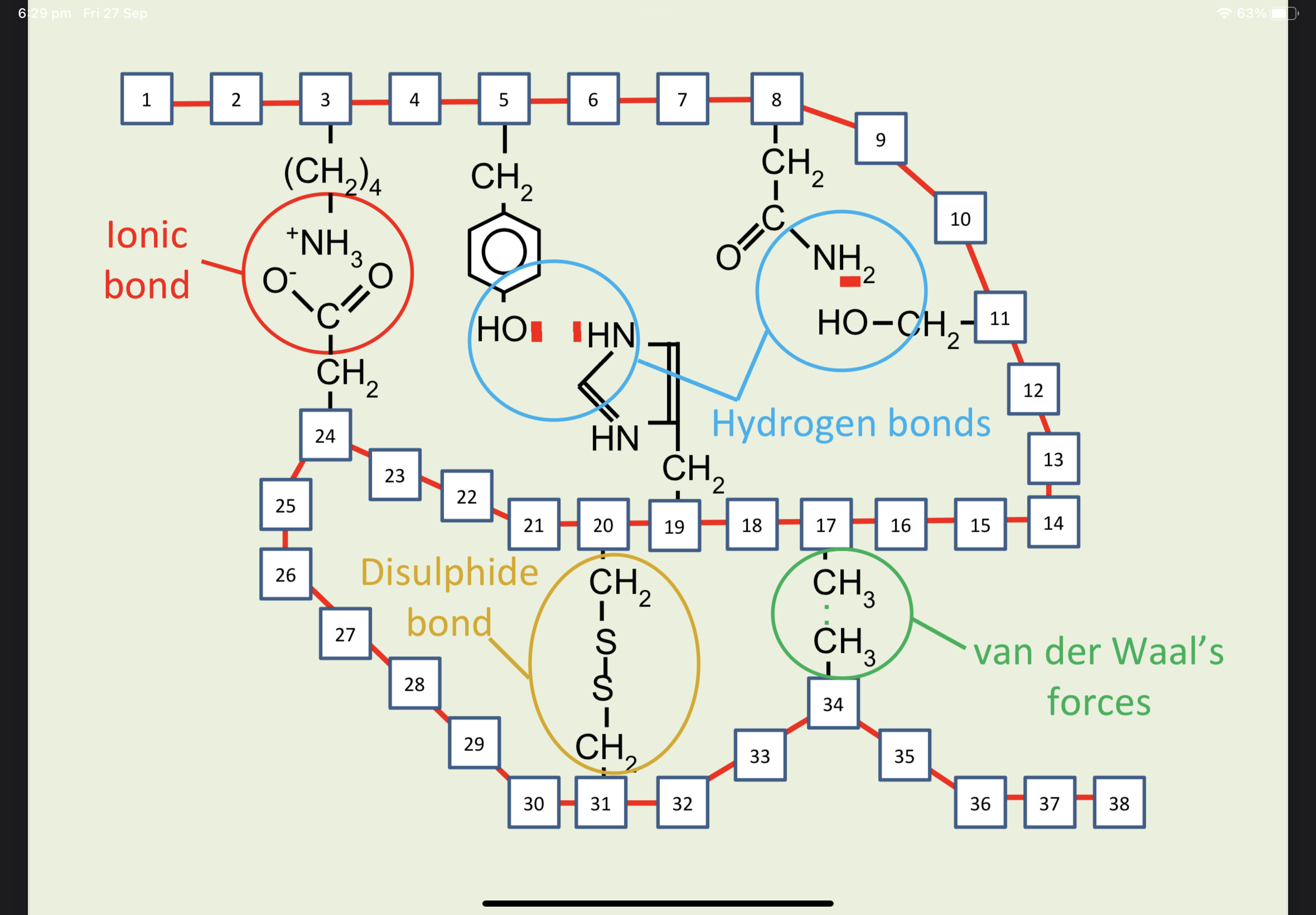
Hydrogen Bonds
Ionic Bonds
Disulphide bonds
Van der Waal's forces
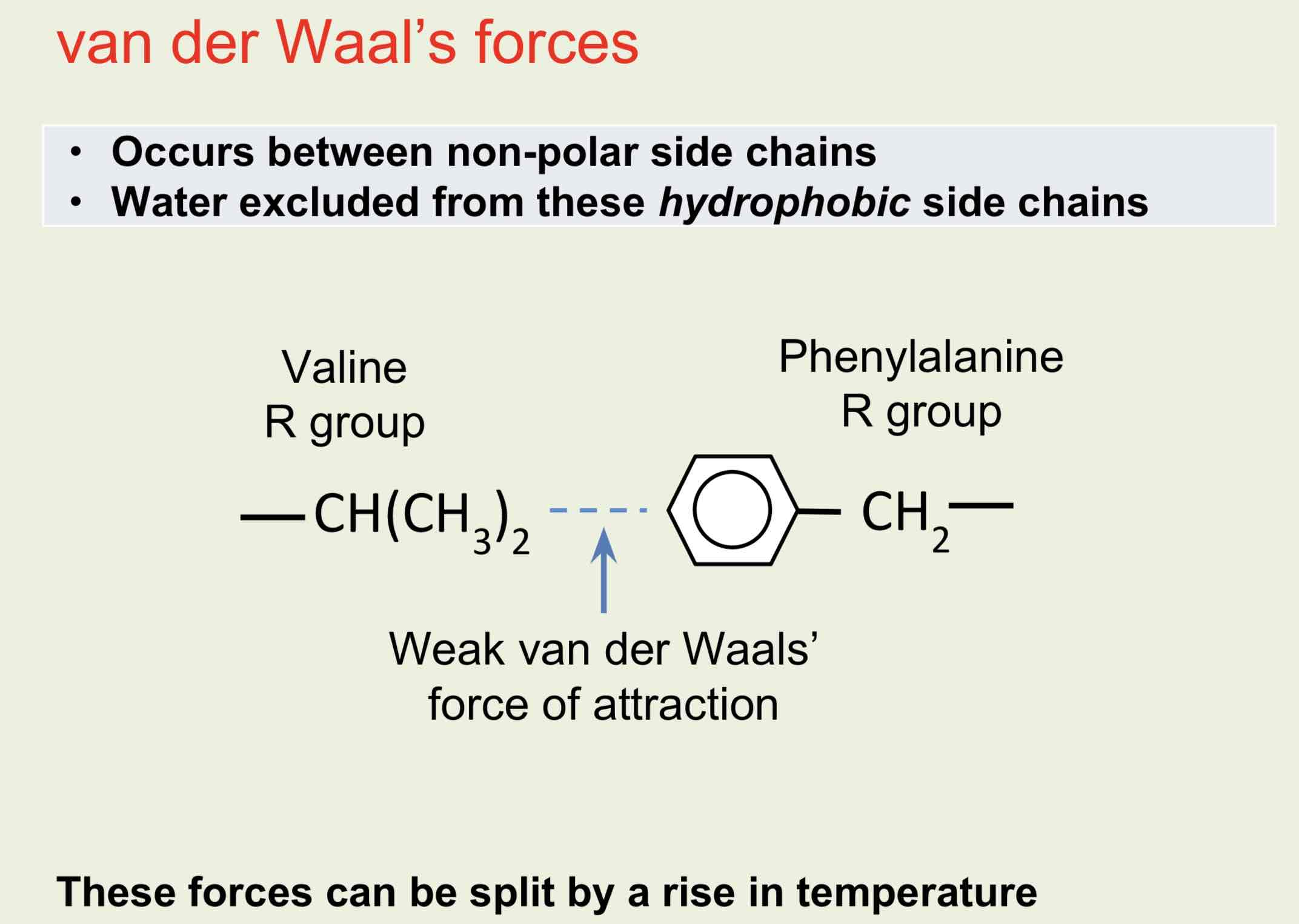
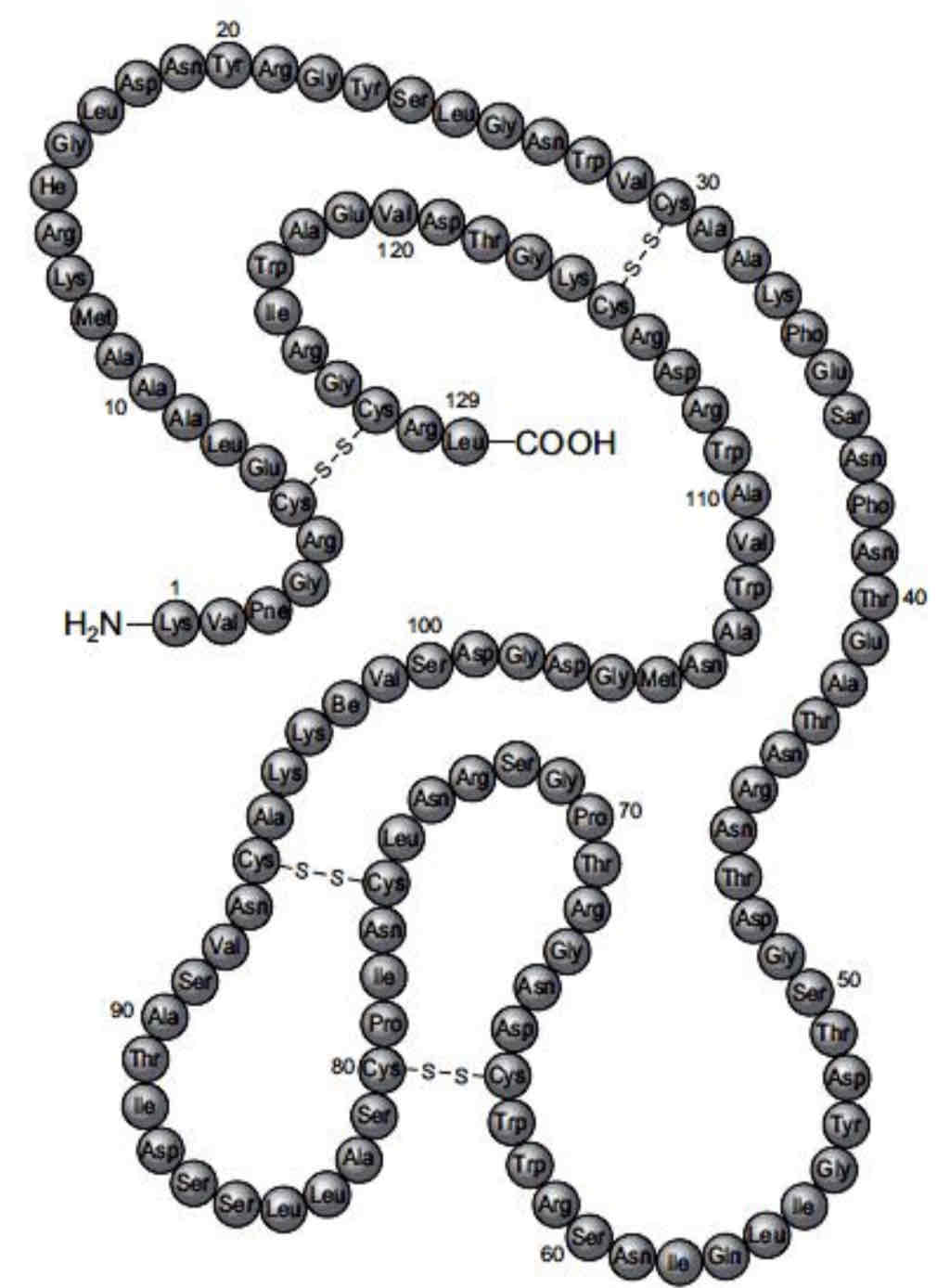
Tertiary Structure
This is the enzyme lysozyme. It has a tertiary structure. The disulphide bonds connect different parts of the polypeptide molecule together. This maintains the shape of the active site, allowing enzyme-substrate complexes to form.
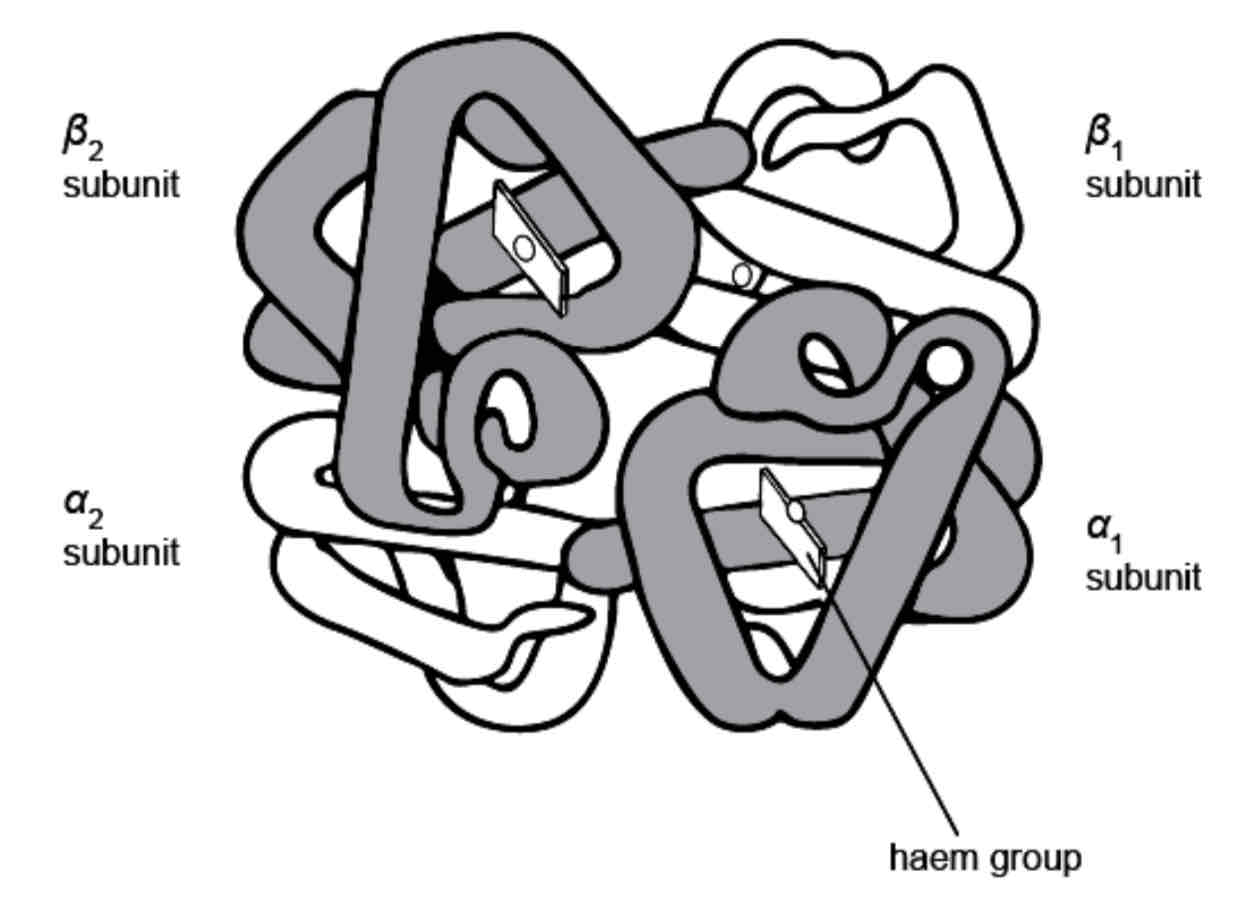
Proteins - Classifications: Globular Proteins
Proteins can be classified into globular and fibrous proteins. Globular proteins have functions such as enzymes, antibodies and hormones. Globular proteins are compact and folded into 3D spherical molecules. They are soluble in water. Haemoglobin is a globular protein (see the diagram); it transports oxygen to the body tissues.
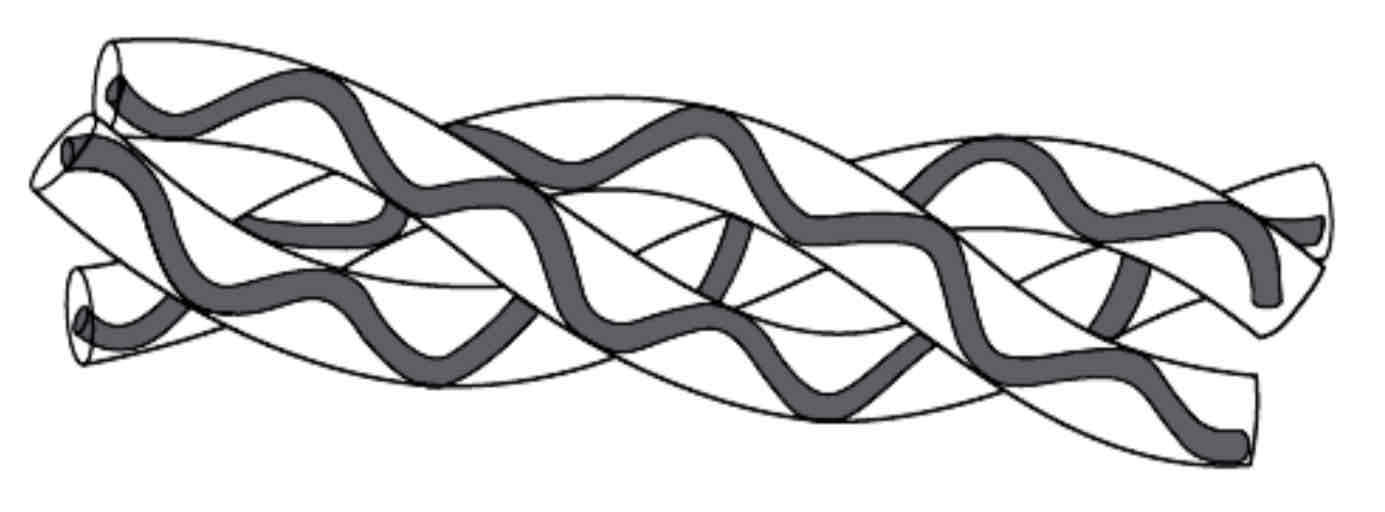
Proteins - Classifications: Fibrous Proteins
Fibrous proteins perform structural functions. They consist of polypeptides in parallel chains or sheets with numerous cross linkages to form long fibres e.g. keratin in hair. Fibrous proteins are insoluble in water, strong and tough.
Collagen (see the diagram) provides the properties needed in tendons (tendons attach muscle to bone); a single fibre consists of three identical polypeptide chains twisted together like a rope. These chains are linked by cross-bridges, making a very stable molecule.
Proteins – Comparing globular and fibrous proteins - Haemoglobin
Haemoglobin:
4 polypeptide molecules
Each polypeptide molecule is different (4 genes are needed to code for haemoglobin)
Haemoglobin is associated with non-protein groups (haem groups)
The highest level of protein structure is quaternary
Proteins – Comparing globular and fibrous proteins - Collagen
Collagen:
3 polypeptide molecules
Each polypeptide is the same (only one gene is needed to code for collagen)
Collagen is not associated with non-protein groups
The highest level of protein structure is secondary
Carbohydrates – Testing for reducing sugars
Sucrose is called a non-reducing sugar because it does not reduce copper ll sulphate.
The Benedict’s test will not work; Benedict’s will remain blue. Sucrose must first be hydrolysed by boiling in dilute hydrochloric acid. Glucose and fructose are formed.
The acid must be neutralised with dilute sodium hydroxide before testing with Benedict’s reagent.
Add Benedict’s and heat/ boil. This should now give a positive result; glucose and fructose are reducing sugars which readily donate an electron to reduce copper II sulphate to form the brick-red/ orange precipitate copper I sulphate.
Lipids – Test for fats and oils
To determine whether a substance contains lipid it is mixed thoroughly with absolute ethanol; any lipid present in the sample will dissolve in the ethanol.
An equal volume of water is added and the sample is shaken. Any dissolved lipids come out of solution, forming an emulsion; this turns the sample cloudy white.
Proteins – Biuret test
The Biuret Test is a chemical test used for detecting the presence of peptide bonds (between amino acids).
In the presence of peptides, a copper ll ion forms a violet/ lilac coloured complex in an alkaline solution (Biuret reagent turns from blue to violet/ lilac).
The intensity of the colour is directly proportional to the protein concentration (or number of peptide bonds).
At low protein concentrations the colour change may not be obvious/ little colour change/ colour change may be masked (a colorimeter will be able to detect the colour change).
Starch Test
Add Iodine Reagent to the Sample
If colour changes from yellow-brown to blue-black, starch is present.
Lipids - What are Triglycerides?
The most common types of lipid are triglycerides; these are the fats and oils.
Like carbohydrates, lipids contain carbon, hydrogen and oxygen atoms (the oxygen content is very low).
Triglycerides are insoluble in water as they are non-polar (hydrophobic); they are soluble in other solvents such as ethanol, chloroform and ether.
How are Triglycerides formed?
Triglycerides are formed by condensation reaction between glycerol and fatty acids. Glycerol is a type of alcohol. Fatty acids are organic molecules which have a –COOH group attached to a long hydro-carbon tail. Three molecules of water are released.
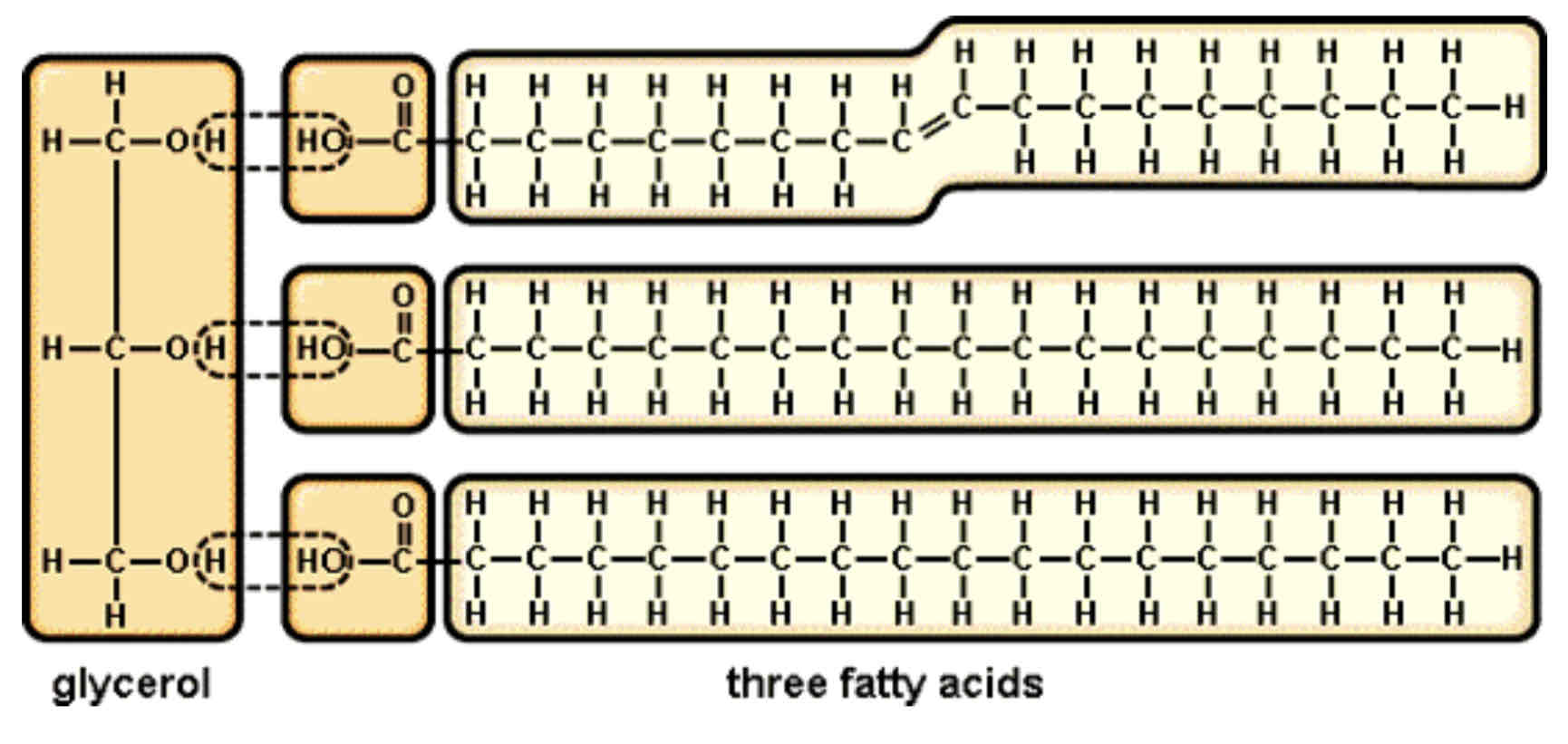
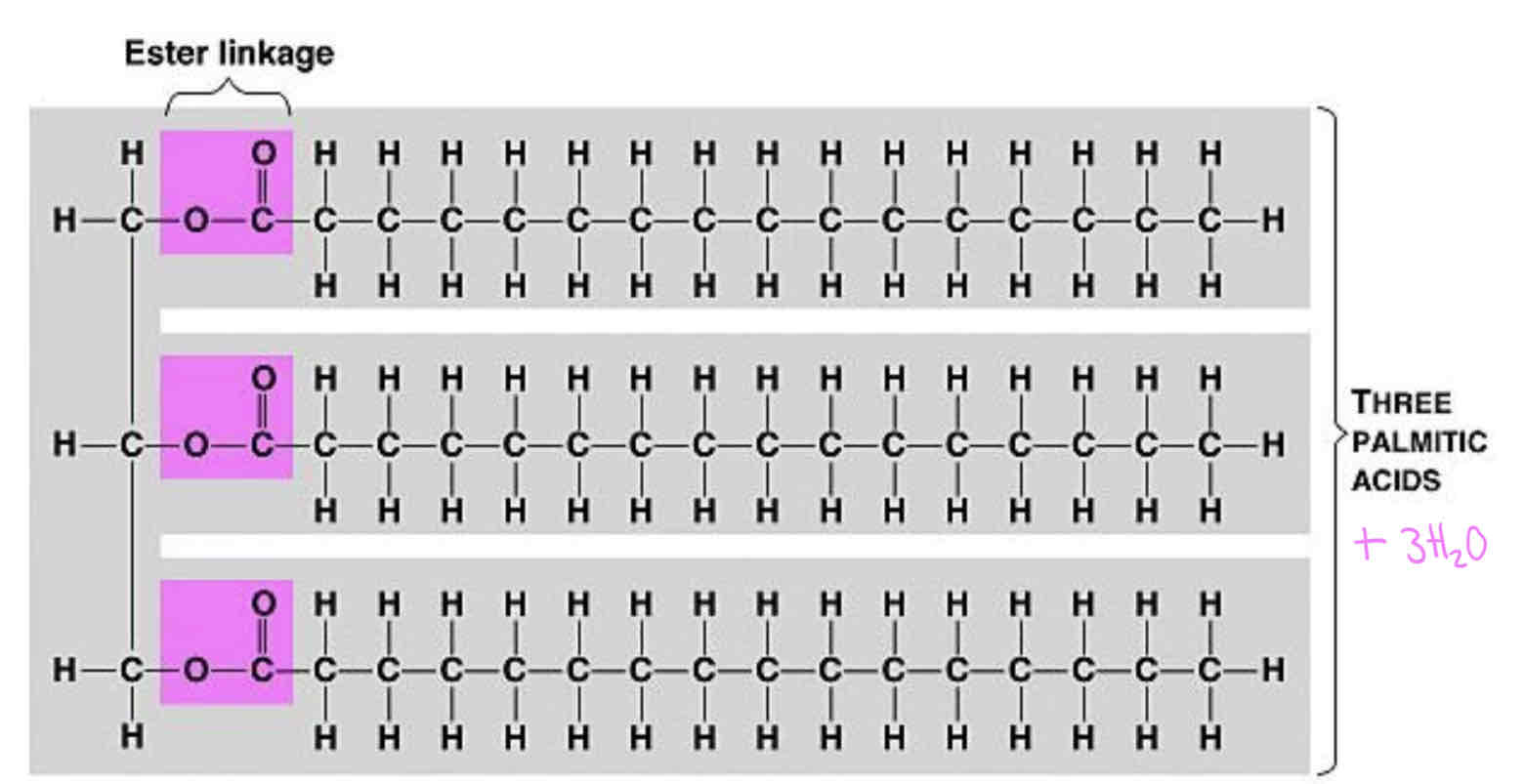
Triglyceride Structure & Bond Formed
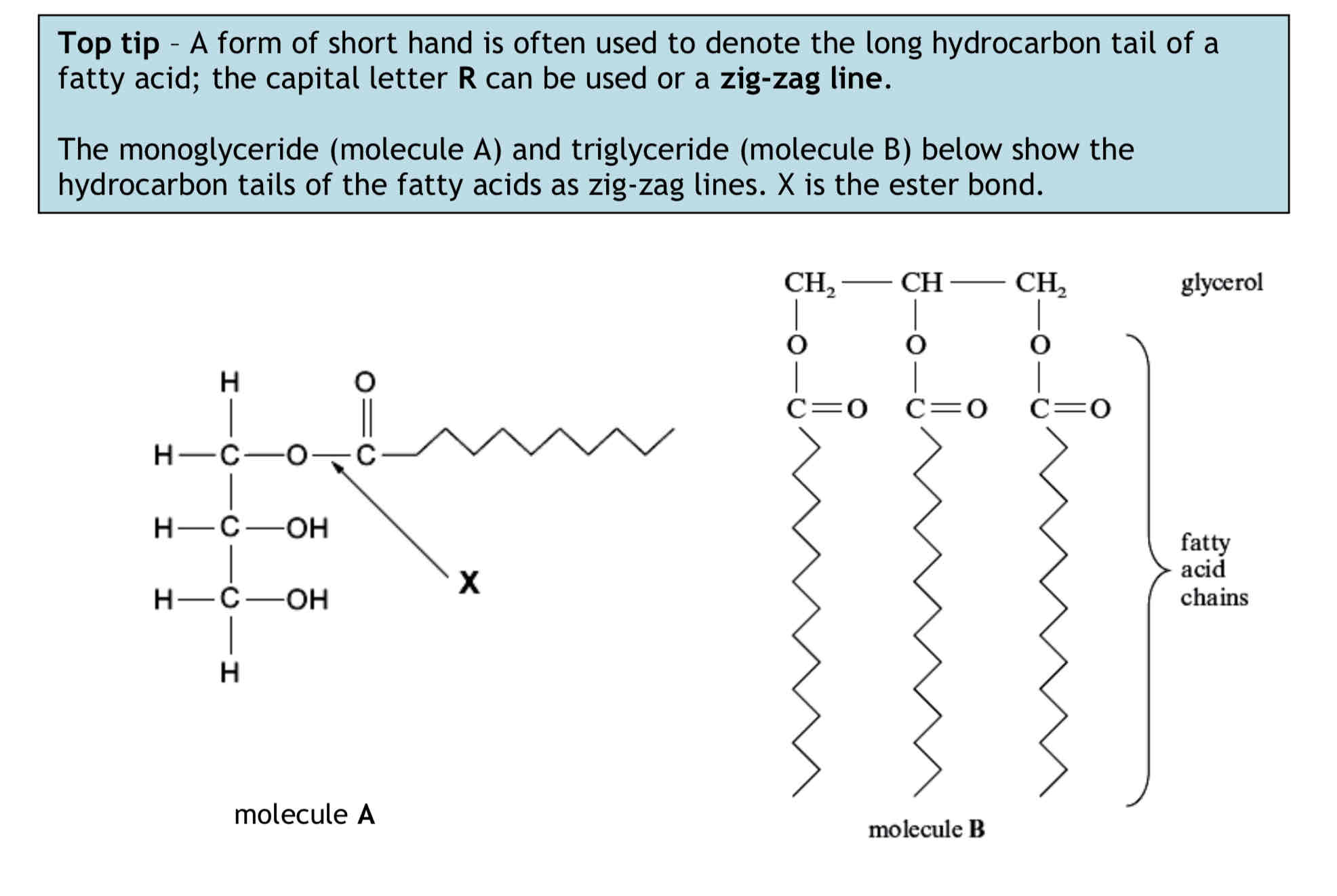
The bond formed is called an ester bond. The ester bond can be broken by hydrolysis. A triglyceride has three ester bonds. (must be able to circle the atoms which make up an ester bond)
Glycerol and fatty acids are not polymers as they have different structures.
Triglyceride Alternative Structure Types
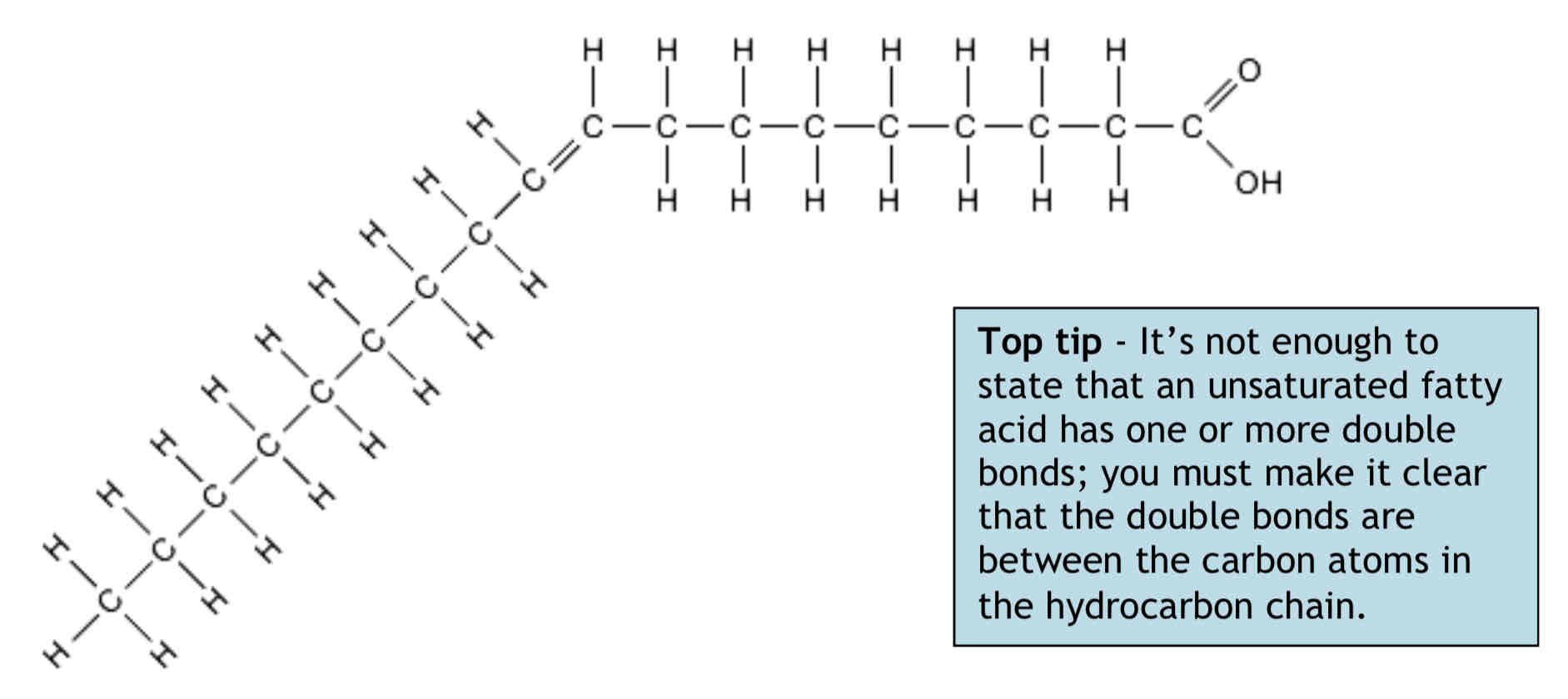
Lipids – What are Unsaturated Fatty acids?
Unsaturated fatty acids have double bonds between neighbouring carbon atoms in the hydrocarbon chain e.g. – C=C-C-C-
Unsaturated fatty acids do not contain the maximum possible number of hydrogen atoms. Double bonds make fatty acids and lipids melt more easily; most oils are unsaturated.
If there is only one double bond between carbon atoms the fatty acid is monounsaturated.
When there are two or more double bonds between carbon atoms the fatty acid is polyunsaturated. A monounsaturated fatty acid is shown below.
Lipids – What are Saturated Fatty acids?
Saturated fatty acids contain only single bonds between Carbon atoms, They have no double bonds between neighbouring carbon atoms in the hydrocarbon tail. A saturated fatty acid carries the maximum possible number of hydrogen atoms. Saturated fatty acids are solid. Animal lipids tend to be saturated. There is a possible link between the consumption of saturated fatty acids and heart disease. A saturated fatty acid is shown below.
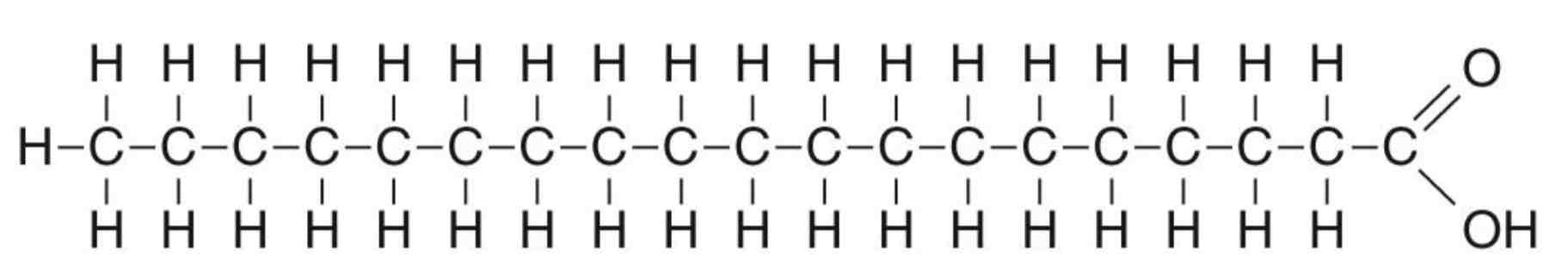
Lipids – Saturated fatty acids and heart disease - What are the main causes of Heart Disease?
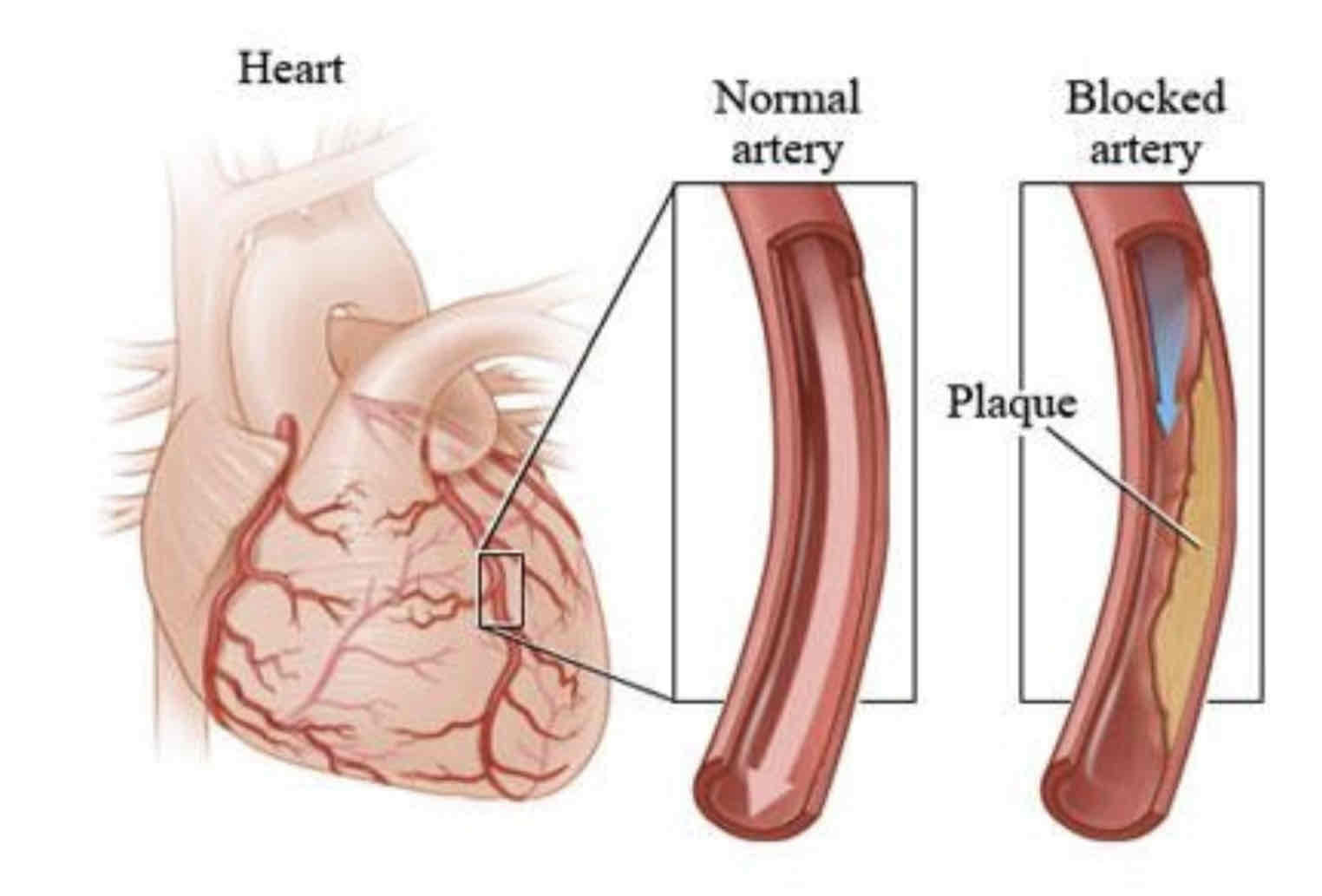
The main causes of heart disease are fatty deposits in the coronary arteries (atherosclerosis) and high blood pressure (hypertension).
A diet that is high in saturated fatty acids, smoking, lack of exercise and ageing are all contributory factors.
What are Lipoproteins & What is their effect?
When food has been absorbed at the small intestine, lipids and proteins combine to make lipoproteins, which travel around the body in the blood stream.
If the diet is high in saturated fats, low-density lipoproteins (LDL) build up. Fatty material called atheroma is deposited in the coronary arteries, restricting blood flow and, therefore, oxygen delivery to the heart tissue. This restricted blood flow can result in angina. If the coronary arteries become completely blocked a myocardial infarction or heart attack occurs.
If the diet has a high proportion of unsaturated fats, the body makes more high- density lipoproteins (HDL), which carry harmful fats to the liver for disposal. The higher the ratio of HDL:LDL in a person’s blood, the lower the risk of cardio- vascular and coronary heart disease.
What does the Inner Wall of the artery have?
The inner wall of the artery has a smooth endothelial lining. Atheroma is deposited on the endothelium, reducing the available volume for blood flow. The atheroma may completely block the artery’s lumen.
What are the functions of Lipids?
Energy reserve (store) in plants and animals
Thermal insulator
Protection
Metabolic water source
Waterproofing
Low density and buoyancy
Nerve Transmission
Steroids and cholesterol
Cell membrane formation
Lipids - Functions - Energy reserve (store) in plants and animals
Triglycerides contain more carbon-hydrogen bonds than carbohydrate.
One gram of fat, when oxidised, yields approximately twice as much energy as the same mass of carbohydrate (e.g) starch.
In animals, fat is stored under the skin and around organs, in plants triglycerides are stored as oils in seeds.
Lipids - Functions - Thermal insulator
When stored under the skin it acts as a thermal insulator which reduces heat loss.
Lipids - Functions - Protection
Fat is often stored around delicate organs such as the kidneys.
Lipids - Functions - Metabolic Water Source
Triglycerides produce a lot of metabolic water when oxidised.
This is essential for desert animals such as the kangaroo rat which never drinks water and survives on metabolic water from its fat intake.
Lipids - Functions - Waterproofing
Fats (being non-polar) are insoluble in water and are important in land organisms such as insects where the waxy cuticle reduces water loss.
Leaves also have a waxy cuticle to reduce water loss by evaporation from the leaf surface.
Lipids - Functions - Low Density & Buoyancy
Fat has a fairly low density and helps animals such as polar bears float in water; it increases their buoyancy. Seeds which store oils can also be easily dispersed as they are light.
Lipids - Functions - Nerve Transmission
Triglycerides form the myelin sheath which surrounds the axon of nerve cells (neurones) in vertebrates; the myelin sheath seeps up nerve transmission.
Lipids - Functions - Steroids & Cholesterol
Steroids, which include the sex hormones (testosterone and oestrogen), are lipids. They have a ring structure rather than a long chain structure.
Lipids - What are Phospholipids? + Structure
Phospholipids are a special type of lipid.
One of the three fatty acid tails is replaced by a phosphate group. The phosphate group is polar and therefore soluble in water.
Phospholipids have hydrophilic heads (attracted to water) and two hydrophobic fatty acid tails (repelled from water).
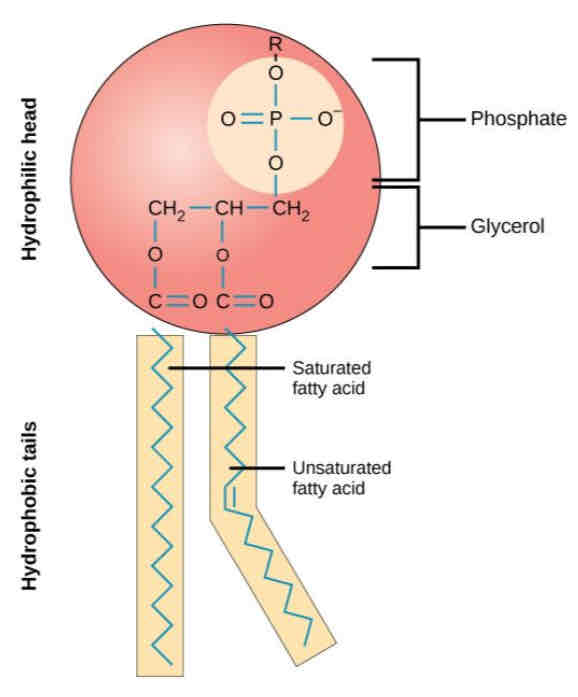
Lipids – Comparing triglycerides and phospholipids
Triglycerides:
3 fatty acid tails
No phosphate group
Non polar (completely hydrophobic)
Phospholipids:
2 fatty acid tails
Phosphate group
Polar head is hydrophilic, fatty acid tails are hydrophobic
Lipids - Functions - Cell Membrane Formation
Phospholipids form a bilayer which is the basis of all cell membranes. The phospholipid bilayer allows for the transport of non-polar molecules across cell membranes by simple diffusion.