epidemiology (lectures 9-13)
1/53
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
54 Terms
randomized control trials
unit of analysis is the individual
one group receives intervention
another group doesn’t
community trails
unit of analysis is group or community
one community receives an intervention
another community doesn’t
natural experiments
unplanned events produce conditions to conduct a natural experiment
levels of exposure differ among populations relative unaffected by other factors, so situation resembles a planned trial
what is an RCT
planned experiment where investigators assign participants to intervention or control condition
test efficacy of intervention or treatment
preventative trials: agent given to healthy or high-risk individuals to prevent disease
therapeutic: treatment/therapy given to diseased to reduce risk of recurrence, improve survival, increase quality of life
has high validity = gold standard in epi
key features of RCT
at least one intervention group, one control group
randomize participants → equal chance of being in intervention or control
blinding to the highest level possible
control group
the comparison arm to gauge effects of treatment
no intervention/placebo/alternative treatment
placebo: positive effect, think they’re getting better even when there’s no physiologic efficacy
good control groups:
have the same baseline
are observed, monitored and followed the same way as intervention group
hawthorne effect: positive effect because they are being observed, change behavior
importance
outline what happens if nothing was done
evaluate how effective/efficacious/safe the treatment is
randomization
want intervention and control groups to look alike in all factors EXCEPT for assigned treatment
patient and physician both don’t know which prevention/therapy is assigned
chance is the only factor that determines group assignment
blinding/masking
groups of randomized individuals aren’t known to anyone involved in the study
used to avoid bias
selection bias
representative sample
information bias
outcomes
three levels of blinding
single-blind: participants are blinded to group assignment, investigators and analysts are aware
protect against placebo effect
double-blind: participants and investigators are blinded to group assignment, analysts are aware
protect against bias to groups from researchers
triple blind: treatment and research aren’t obvious to participants or investigators, analyses are completed where analysts are blinded from group assignment
conducting an RCT
select intervention and develop proposal
ethics committee review
assemble cohort
measure baseline variables
choose comparison groups
monitor participants for outcome
ensure compliance
selective intervention
start with research objective
establish if therapies are to be tested:
safe?
active against disease?
superior to other treatments?
feasible in terms of implementation?
assembling trial cohort
inclusion criteria: population best for generalizing findings, population most efficient for answering the question
exclusion criteria: populations that inhibit ability to control errors, population that has difficulty with therapy compliance
measuring baseline variables
measured to characterize study cohort
demographics
clinical factors of relevance
final report displays baseline characteristics of intervention vs. control group
selecting outcomes
outcome: endpoint or dependent variables
remission
stopping cancer growth
growth of cancer
mortality
selection of best endpoint may be complicated → choose surrogate or intermediate endpoints
assuring compliance
must monitor adherence to intervention protocol
self reports, direct evaluation, pill counts, metabolite levels
easier to comply with once-a-day treatment
monitor attendance and engagement (behavioral interventions)
analyzing results
report overall risk and rates
present comparison of risk and rates of outcomes between groups
present relative risk of outcome in exposed vs. unexposed groups
measure quality of life interventions or severity of symptoms
measuring associations: risk
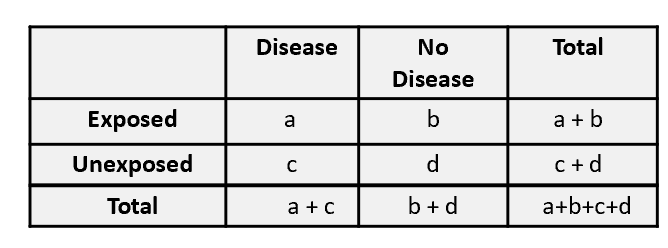
risk ratio: a/(a+b)/c/(c+d)
measuring associations: rates
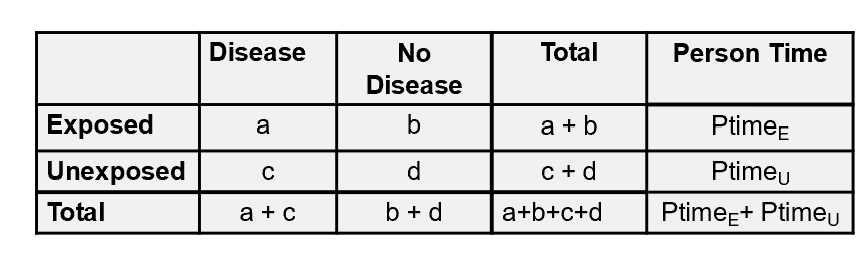
rate ratio: a/person timeE/c/person timeU
ethical considerations
chemical equipoise: balance doubt and efficacy of treatment with belief it may work
if research know new treatment is beneficial or toxic, trail should stop
beneficial to control group
toxic to intervention group
Data Safety and Monitoring Boards (DSMB): monitor trial data to make determinations
advantages of RCT
investigator controls amount, timing and frequency of exposures
demonstrate cause-effect relationships
randomization and blinding ensure high validity
only practical approach for research questions
disadvantages of RCT
cannot study harmful materials or methods
interventions may not be suitable for blinding are are different from real world practice
limited generalizability
difficulty following people over time, miss data for outcomes
very expensive
hard to recruit participants
types of trials in pharmaceuticas
non-inferiority
new treatment is as effective as standard of care
test whether treatments are similar
null hypothesis: treatments are different
superiority
new treatment is better than standard of care
null hypothesis: new treatment is better than standard of care
phases of RCT in pharmaceuticals
phase 0: learn how drug is processed and affects the body
phase 1: find best dose with fewest side effects
phase 2: safety and efficacy
phase 3: compare new drug to standard of care
phase 4: test new drugs approved by FDA