optical isomerism
1/7
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
8 Terms
what is optical isomerism? when does it occur?
form of stereoisomerism
occurs as a result of chirality in molecules
limited to molecules w/ a single chiral centre
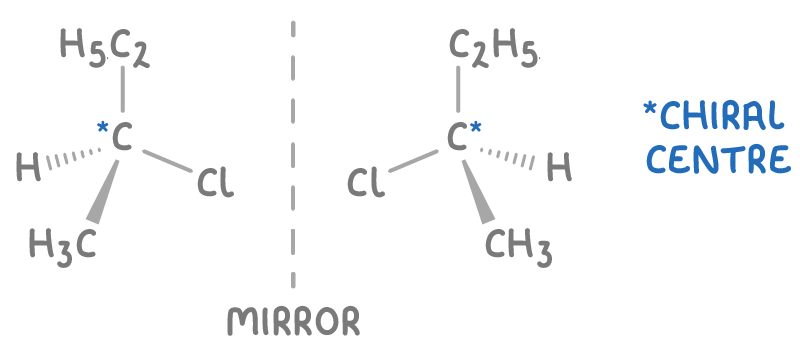
what is a chiral C?
C with 4 diff groups attached
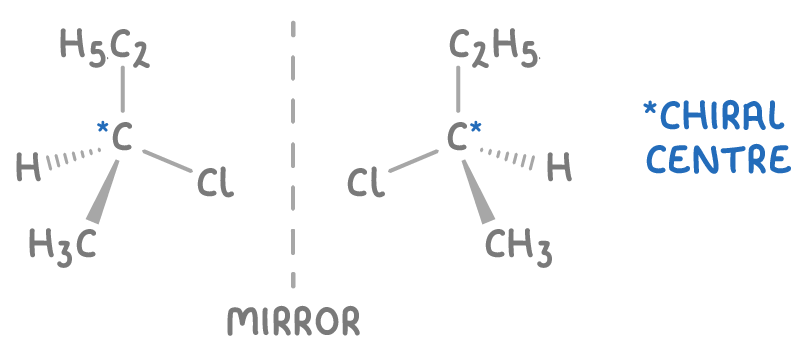
what is an enantiomer?
isomers where groups are arranged around chiral Cs differently
are non superimposable mirror images of each other
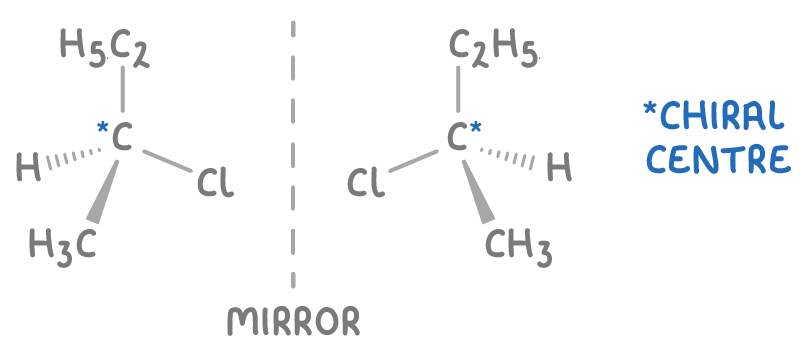
what is a racemic mixture/racemate?
mixture of equal amounts of enantiomers
how are enantiomers similar and how do they differ?
have identical chemical and physical properties
but differ in their effect on plane polarised light (as they are optically active) and their reactions w/ other chiral molecules
describe the effect of enantiomers on plane polarised light:
enantiomers are optically active - rotate plane polarised light
plane polarised light typically only vibrates in one direction
the 2 enantiomers will rotate the plane of polarised light in opposite directions (i.e. clockwise and anticlockwise)
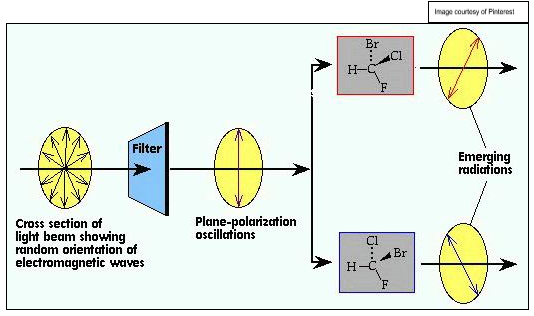
give and explain the rotational effect on plane polarised light of a racemic mixture:
overall effect of zero
opposing rotations cancel out
so racemic mixture is optically inactive
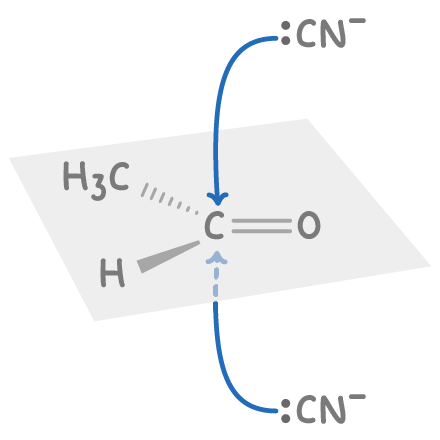
explain how reactions involving carbonyl groups often produce racemic mixtures:
carbonyl is planar around functional group
∴ attack from either either side =lly likely
giving = amounts of both enantiomers