Chemistry (CH.1)
1/62
Earn XP
Description and Tags
Basic definitions, terms, and other things needed that we discussed within the first week
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
63 Terms
Chemistry
The study of what substances are made of and how they behave
Matter
Anything that has mass and takes up volume
Atom
The fundamental unit of matter
Element
Made of only one type of atom
Compound
Composed of more than one element, bound in a fixed ratio
Molecules
Groups of atoms that bind tightly together and behave as a single unit
Diatomic Molecules
Molecules made up of just two atoms, chemically bonded together
Pure substances
Contains only one type of element or compound
Mixture
contains more than one substance, not bound in a fixed ratio
Bronze is an example of…
…a mixture of copper and tin
Homogeneous Mixtures
Components are mixed evenly and appear to be uniform throughout
Heterogeneous Mixtures
Components do not mix evenly
Sand and water, when combined is an example of a…
heterogeneous mixture
Salt and water, when combined is an example of a…
homogeneous mixture
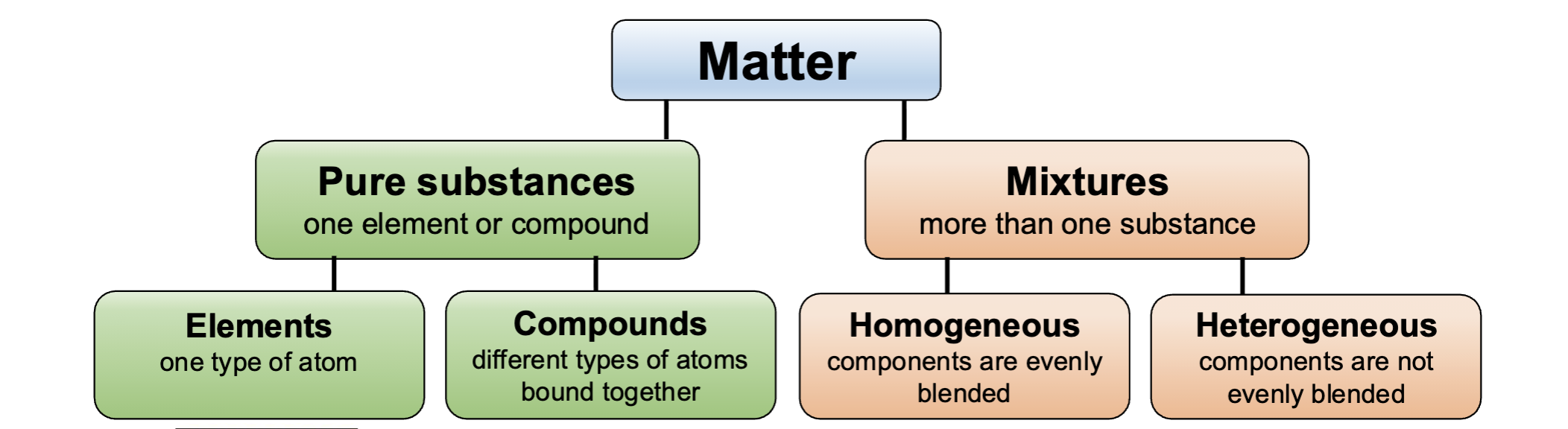
atoms → elements and compounds → pure substances →matter← mixtures←homogeneous and heterogeneous
Gatorade is an example of …
a homogeneous mixture
Crystalline sugar is an example of…
a pure substance
Led wire is an example of …
a pure substance
Salsa is an example of
a heterogeneous mixture
Solid to Liquid
Melting
Liquid to Gas
Vaporization
Solid to Gas
Sublimation
Gas to Liquid
Condensation
Liquid to Solid
Freezing
Gas to Solid
Deposition
Physical Properties
Can be measured without changing the identity of the substance
What are the five main physical properties?
Color, Hardness, Mass, Temperature, and Volume
Physical changes
don’t change the identity of the substance
Chemical Properties
Can NOT be measured without changing the identity of the substance
Chemical Changes
Change the identity of the substance — also called chemical reactions
Elements combining to form compounds is an example of a …
chemical change
A change that forms new compounds is…
a chemical change
Intensive properties
do not depend on the amount of matter in a sample
(I, do not depend)
Extensive properties
depend on how much matter a sample contains
Examples of Intensive Properties
Boiling point, concentration, temperature, luster
Examples of Extensive Properties
Entropy, Length, Weight, Volume
Units of measurement
quantities with accepted values that can be communicated between people
SI Units
The relationship between some common english and metric units
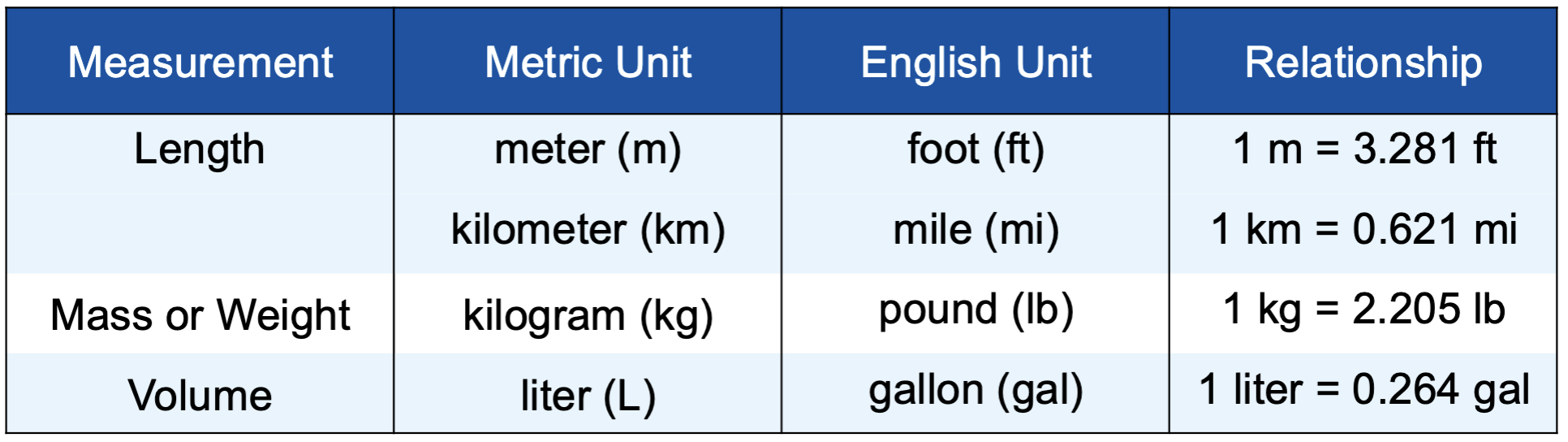
Mass
Kilograms (kg)
Length
Meters (m)
Time
Seconds (s)
Temperature
Kelvin (K)
Amount
Moles (mol)
Light Intensity
Candela (cd)
Electric Current
Ampere (A)
Volume
m³
Velocity
m/s
Density
kg/m³
tera-, T
1012, 1,000,000,000,000
giga-, G
109, 1,000,000,000
Mega -, M
106,1,000,000
Kilo-, k
10³,1,000
Deci-, d
10-1, 1/10
Centi-, c
10-2,1/100
Milli-, m
10-3,1/1,000
Micro-, μ
10-6, 1/1,000,000
Nano-, n
10-9, 1/1,000,000,000
Pico-, p
10-12, 1/1,000,000,000,000
Boiling point of water
212 F, 100 C, 373 K
Freezing point of water
32 F, 0 C, 273 K
Absolute Zero
-460F, -273C, 0K
Equation for Fahrenheit
F= 9/5 C+32
Equation for Kelvins
K= C+ 273.15