Nucleophilic Substitution
1/42
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
43 Terms
What is a concerted reaction?
All bonds are made and broken at the same time
What makes a good leaving group for SN2?
Weak C-X bonds, happy to accept electrons
Low pKa values (counter ions of strong acids)
How does acidity of a hydrophilic acid affect leaving group ability?
More acidic the hydrophilic acid, the better the leaving group
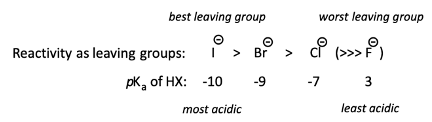
Is hydroxide a good leaving group?
No - conjugate acid of hydroxide is water (not acidic)
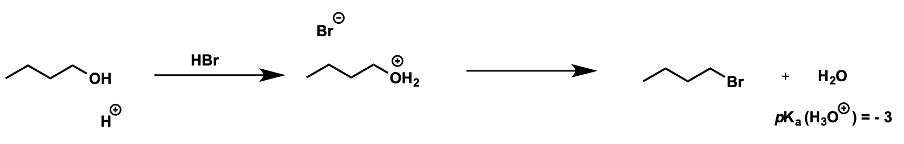
why can alcohols be activated by protonation?
what must the nucleophile be?
leaving group is water
low pKa -3
nucleophile must be non basic
Why can’t a base be used to activate alcohols?
It would react with the acid in a neutralisation reaction
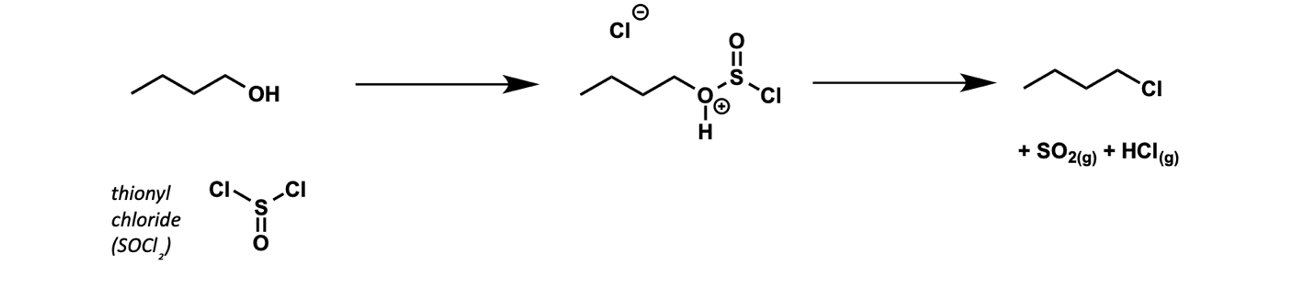
how can alcohols be chlorinated?
with SOCl2
how can alcohols be brominated?
with PBr3
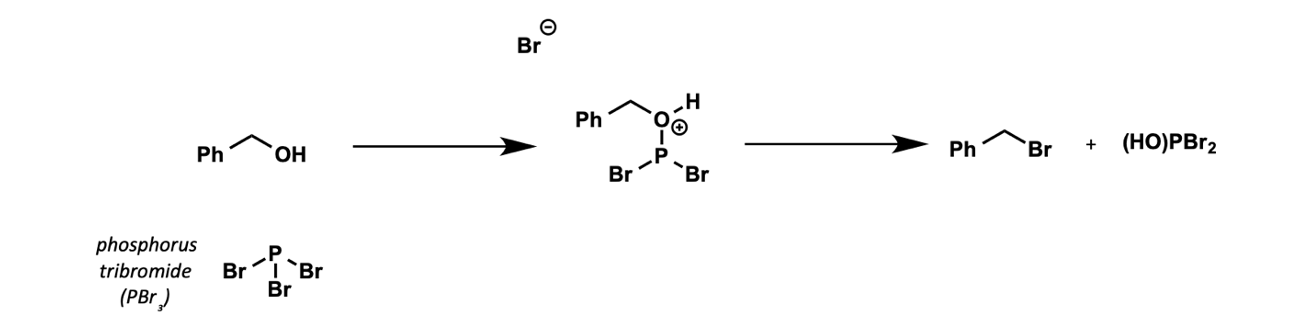
What can alcohol be converted to to make a better reagent?
Haloalkanes
Sulfonate ester
why would alcohols be converted to sulfonate esters?
sulfonic acids are very strong acids (pKa -3)
sulfonate anions are good LGs
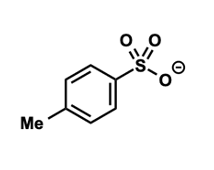
what is a tosylate group?
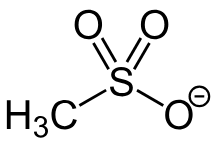
what is a mesylate group?
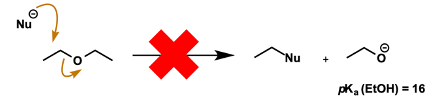
Why are alkoxides not good leaving groups?
Therefore what type of molecules cannot be electrophiles?
Conjugate acids are alcohols (high pKa)
ethers
What is the only type of ether than can be used as electrophiles?
Cyclic three-membered ethers (epoxides)
Why can epoxides be used as electrophiles?
C-O is weak because of angle strain
when epoxide opens, the relief of the angle strain provides extra driving force to overcome otherwise unfavourable formation of epoxide
sp3 carbons want angle of 109.5, so more angle strain with 60, so springs back to 109.5 when released
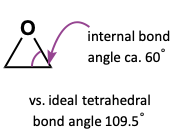
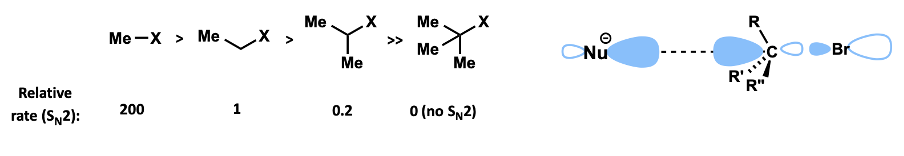
How does reactivity in SN2 change with increasing substitution around carbon undergoing attack?
reactivity decreases with more substitution
methyl > primary > secondary > tertiary alkyl group
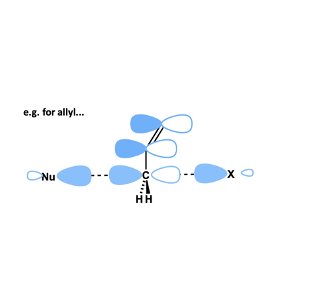
How is rate of SN2 affected by presence of neighbouring π-systems on electrophile (alkenes, aromatics, carbonyl groups)?
rate is increased substantially
stabilised by overlap of π-system with the ‘p-orbital’ on carbon in the transition state
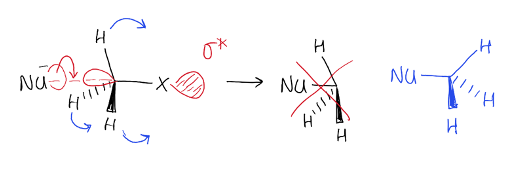
What happens to bonds on carbon being attacked during sn2?
Bonds flipped to the other side
Opposite stereochemistry to starting material
e.g. Nu ends up on opposite side of stereogenic centre from where Br was
What is the ratio of enantiomers formed during SN1?
1:1
Why are both enantiomers formed in sn1 reactions?
The stereochemistry does not have to invert
stereochemical origin of starting material is lost in planar carbocation - equal likelihood of attach from either face
What are characteristics of HOMO for good nucleophile?
high lying HOMO (readily donatable pair of electrons) for effective overlap with LUMO of electrophile (C-X σ* orbital)
How does basicity affect ability of nucleophile?
for a given element, the more basic the species, the better the nucleophile
How is nucleophilicity affected by structural effects e.g size?
The bigger a nucleophile, the poorer it is
harder to approach the σ* orbital
What happens if the good SN2 nucleophile is also a good base? e.g. hydroxide
competing E2 elimination reaction can occur
balance between SN2 and E2 depends on how good of an SN2 electrophile the other reactant is (slower SN2 means E2 dominates)
How to prepare phenoxide anions?
Treat phenol with a base to deprotonate it
Does SN2 or E2 dominate when the nucleophile and electrophile are non hindered? How does increasing hindrance affect this?
SN2 dominates for non hindered
Increasing hindrance on either increases E2
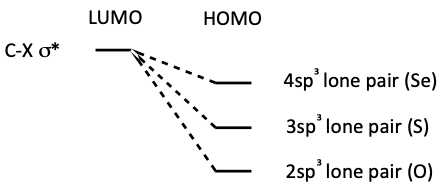
How does nucleophilicity change down a group for thiolates?
why?
Nucleophilicity increases
higher energy HOMO better matched to LUMO
Why is cyanide anion a good SN2 nucleophile?
Small and negatively charged on carbon
why are carboxylate anions favourable for Sn2 reactions?
not basic as counter ions of weak acids
don’t cause issues with E2 and only SN2 observed
why are phenoxides favourable for SN2 reactions?
good nucleophiles
only weakly basic so no issues with E2
what can alkoxides be used for in SN2 reactions?
how can they be prepared?
what is a problem with this?
good nucleophiles
anions need to be preformed as alcohols not acidic
need relatively strong base e.g. NaH
they are quite basic and so can be issues with E2
what are thiolates?
R-S-
why can hydroxide be used to make thiolates but not alkoxides?
thiols are more acidic than alcohols so hydroxide is fine as a base
are thiolates better or worse nucleophiles than oxygen equivalents?
better
what is the problem with amines as nucleophiles?
hard to stop the reaction after substitution as they are such good nucleophiles
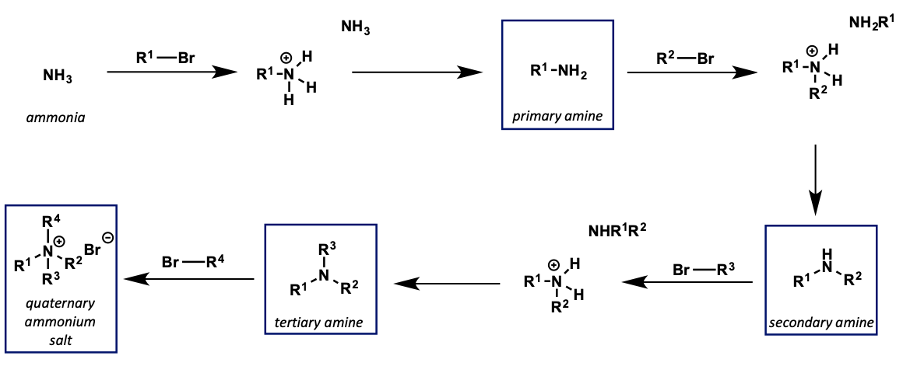
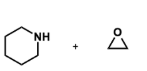
why are epoxides used with amines in SN2 reactions?
nucleophilicity of amino alcohol product is reduced by hydrogen bonding
reaction is more controlled in terms of over alkylation
what are azides?
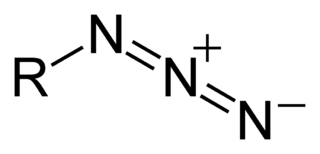
why are azides good nucleophiles?
small, linear molecule with negative charge on nitrogen
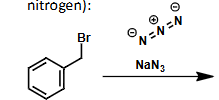
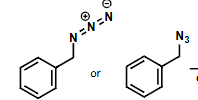
what is the product?
how can an azide be reduced?
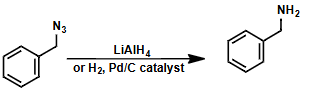
why is overalkylation not possible with azide nucleophiles?
reduced to give primary amine
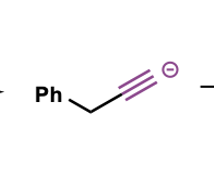
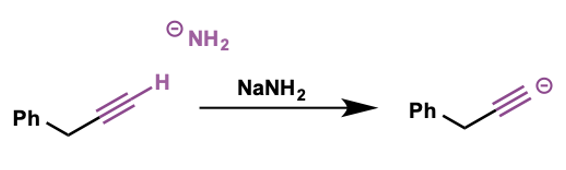
how can acetylide anions be made?
treat alkyne group with strong base such as NaNH2
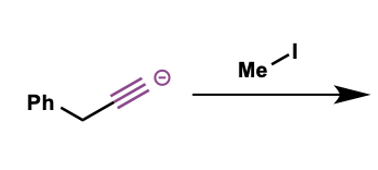
what are the products of acetylide anions used as nucleophiles?
what would be the products?
E and Z alkenes