chemical reactions
1/31
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
32 Terms
What are some examples of a chemical reaction?
Some examples are: Digesting food, Frying an egg, exploding fireworks and the rusting of iron.
What happens in a chemical reaction?
In a chemical reaction, one or more substances (the reactants) are changed into one or more different substances (the product)
What happens always happens in a chemical reaction?
A new substance is always formed
What was the aim for the experiment where we heated a solid?
The aim was to find out signs of a chemical reaction when copper carbonate (green colour) was heated strongly.
What are signs of a chemical reaction?
some signs are: A gas is given off, colour change, a solid is formed (precipitate), a new substance is formed, energy changes (heat taken in, or given out)
What was the method used for the experiment where we heated a solid?
We set up the clamp stand before placing the boiling tube into the clamp
Then, we added 2-3 spatulas of copper carbonate into the boiling tube
Finally, we turned on the Bunsen burner to heat the copper carbonate strongly
What was the result of heating copper carbonate strongly?
When copper carbonate (the reactant) is heated strongly, it changes into copper oxide (black colour) and carbon dioxide. (The product)
What does it mean if a compound ends in -ate?
When a compound ends in -ate, it means that oxygen is present in the mixture
What was the conclusion from the 'heating a solid' experiment?
Heating copper carbonate is a chemical reaction.
What was the aim of the 'mixing solutions' experiment?
The aim of the experiment was to find the signs of a chemical reaction when solution A (Copper Nitrate) and solution B (ammonium carbonate) are mixed together.
What does solution A (copper nitrate) look like?
Solution A is blue and clear.
What does solution B (ammonium carbonate) look like?
Solution B is colourless and clear.
What was the method for the 'mixing solutions' experiment?
Place a test tube rack on your station
Grab 2 test tubes and place them into the rack
Pour 3ml of Solution A (copper nitrate) into one test tube, and 3ml of solution B (ammonium carbonate) into the other test tube.
Then, mix solution A and B together
What was the result of the mixing solutions experiment?
When Copper nitrate (blue and clear) and ammonium carbonate (colourless, clear) are mixed together, it turns into copper carbonate (precipitate) and ammonium nitrate (clear solution.
What is the conclusion of the mixing solutions experiment?
When solution A ( copper nitrate) and solution B (ammonium carbonate) are mixed together: A new substance is formed, and a precipitate is formed
What happens when a precipitate is formed?
When a precipitate (a solid) is formed, it is called a precipitation reaction
What is an exothermic reaction?
An exothermic reaction is when a reaction gives heat out, causing the temperature to increase.
What is an endothermic reacton?
An endothermic reaction is when heat gets taken in, causing the temperature to decrease
What happens when hydrogen is burned?
When hydrogen is burned, sound energy and light energy is produced when the balloon filled with hydrogen was burned.
What are some examples of changes in appearance in a chemical reaction?
Colour change, a gas given off, or a solid forming (precipitate)
What are some examples of energy changes?
Energy changes include making light, sound and heat being given out (exothermic reaction), or heat being taken in (endothermic reaction)
What are all substances made up of?
All substances are made up of very tiny particles called atoms
What are elements?
Elements are the simplest kind of substance which contain only one type of atom.
What are some examples of different types of atoms?
Oxygen Atoms, Helium Atoms, hydrogen atoms

What are mixtures?
Mixtures are elements which are not chemically joined, so they are easy to separate.
What are compounds?
Compounds are made up of atoms which are chemically joined by bonds. This makes it hard to separate them.
what is iron?
Iron is a transition metal which is silver in colour, and it is magnetic.
What is sulphur?
Sulphur is a non metal which is yellow in colour, and it isn’t magnetic.
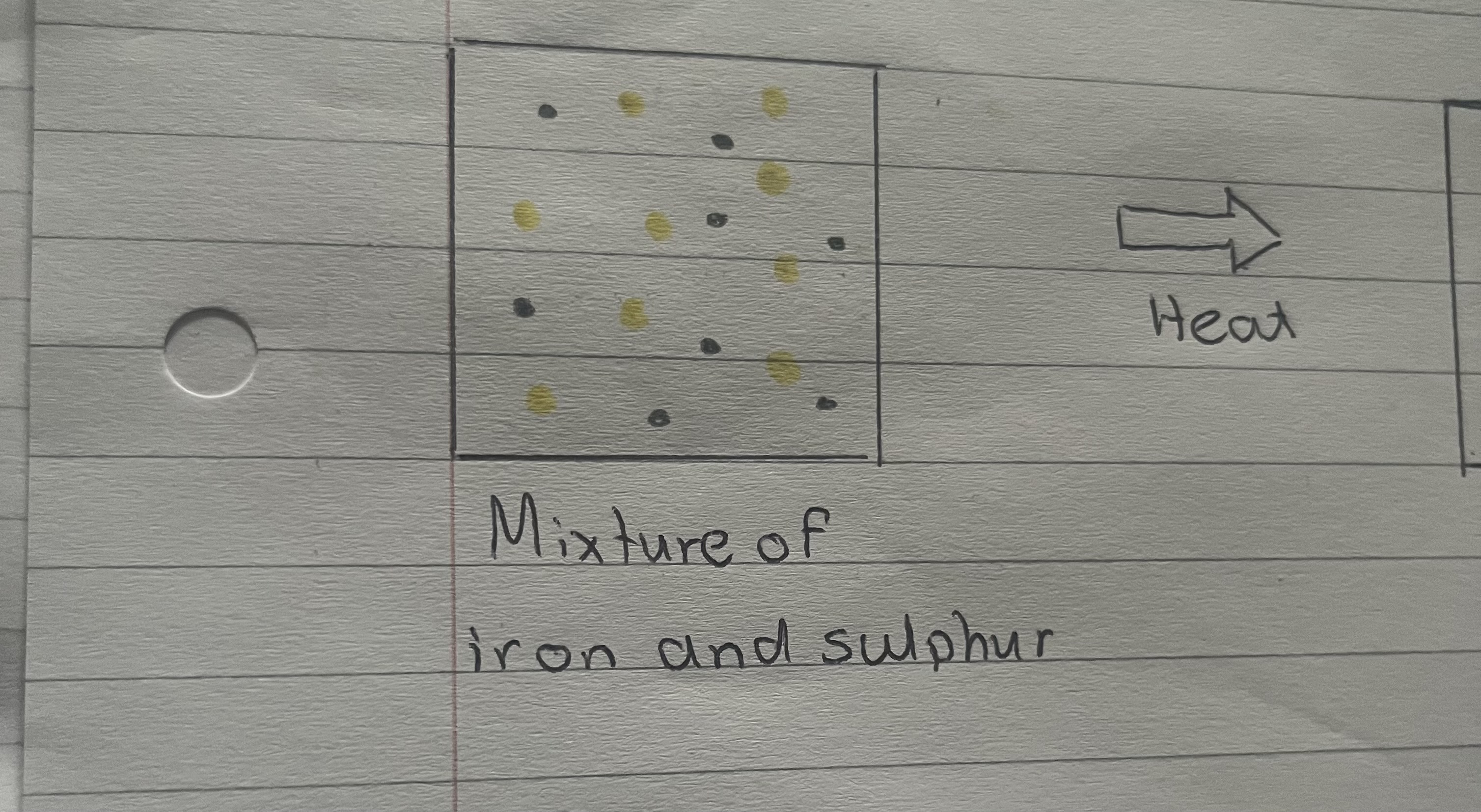
What happens when you mix iron and sulphur together?
When iron and sulphur is mixed together, the iron can easily be separated using a magnet, as mixtures are easily separated
What happens when you heat up iron and sulphur?
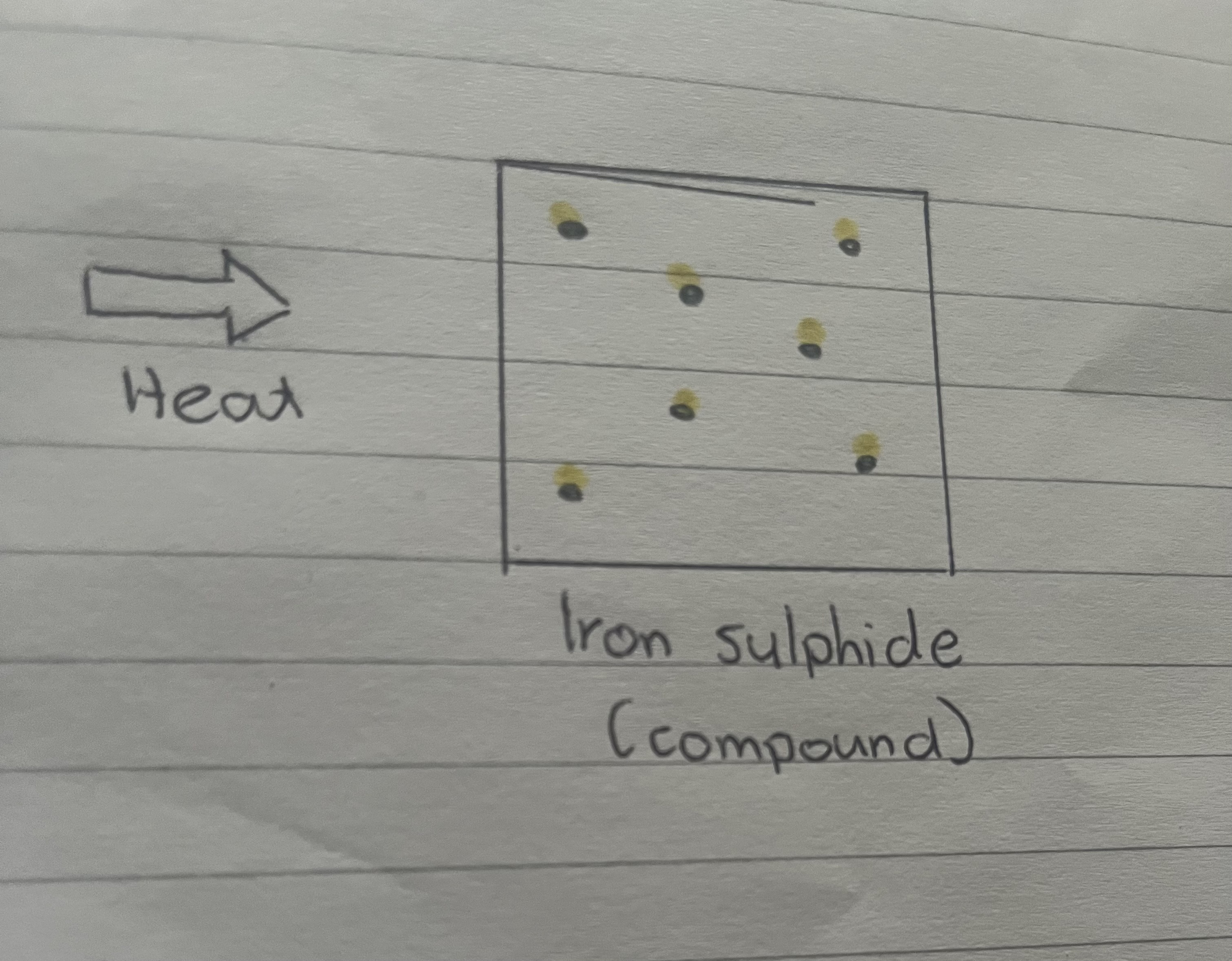
When you heat up the iron and sulphur mixture strongly using a Bunsen burner, the compound iron sulphide is formed which is not magnetic, and it cannot be separated.
What does a mixture of iron and sulphur look like?
What does a compound of iron sulphide look like?