1.20 - innate immunity 2
1/49
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
50 Terms
what is the complement system?
what does it do?
how is it activated?

what are the 3 pathways of complement activation?
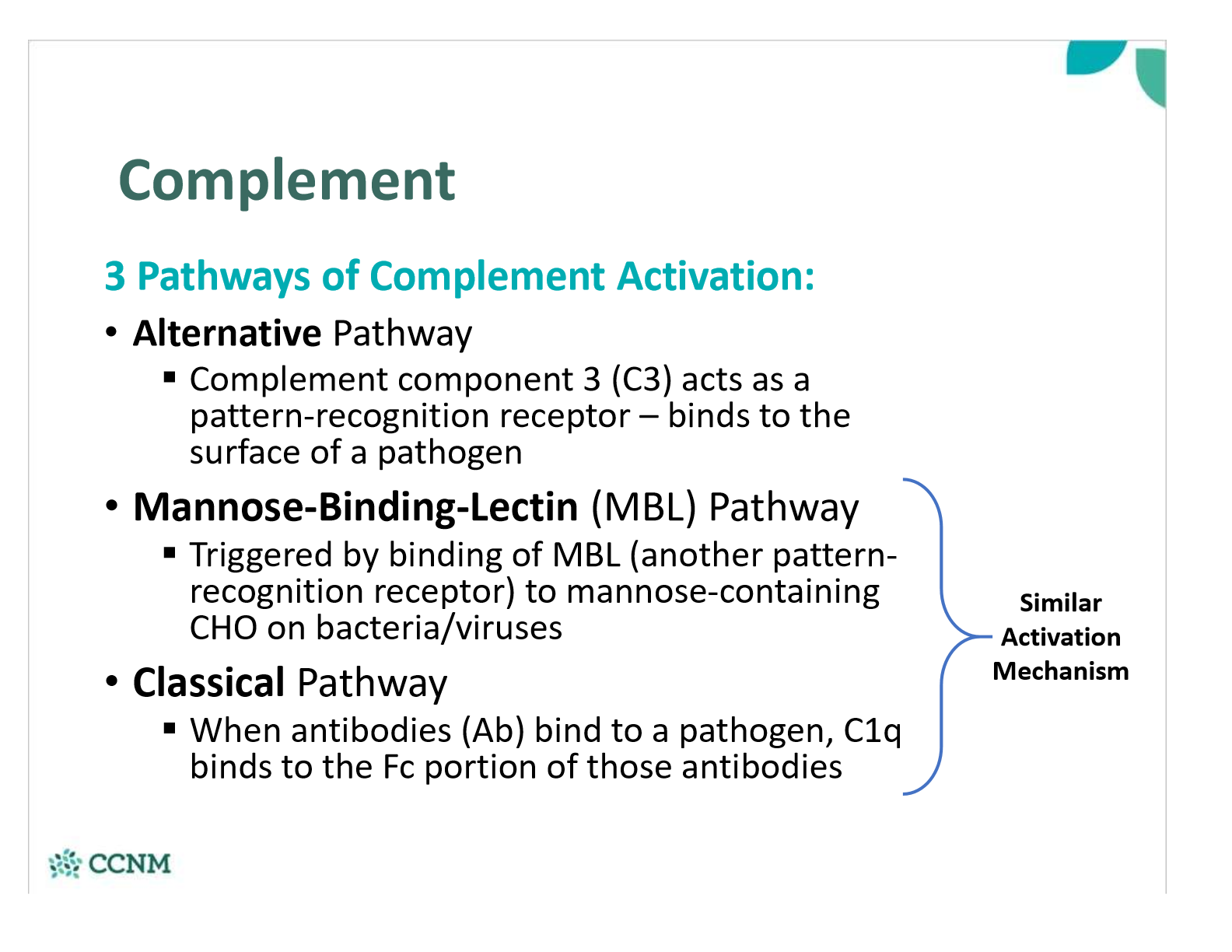
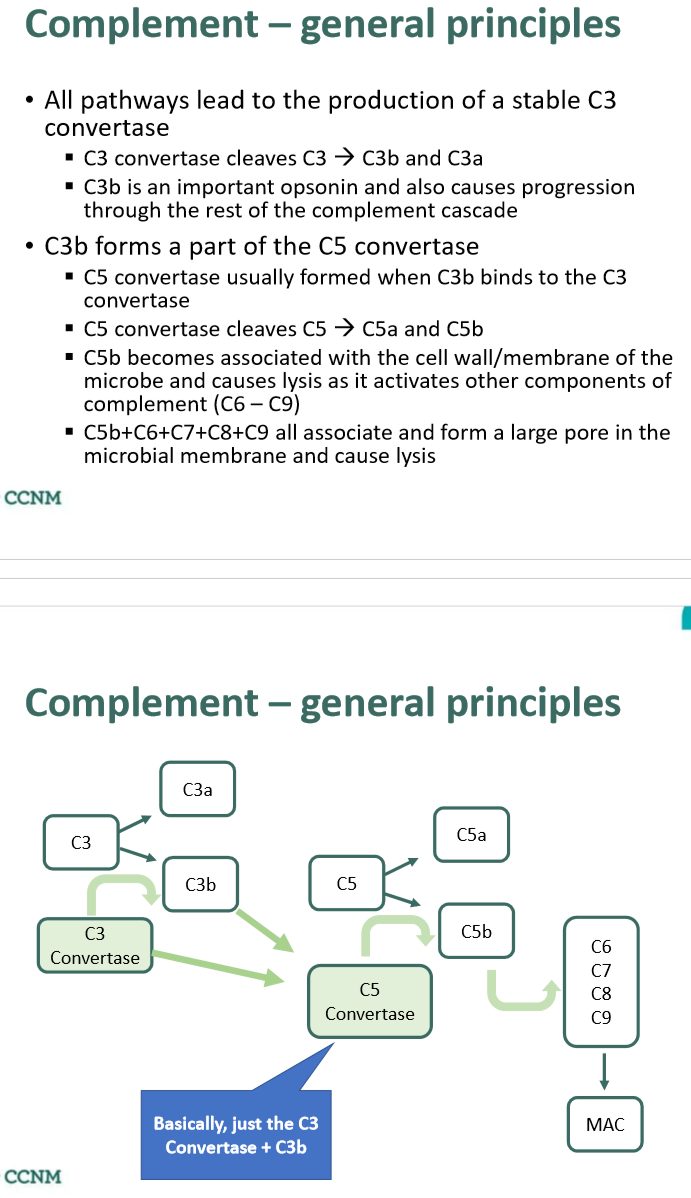
what is the main mechanism of the complement system?
in the alternative pathway, what happens to C3 in the blood?
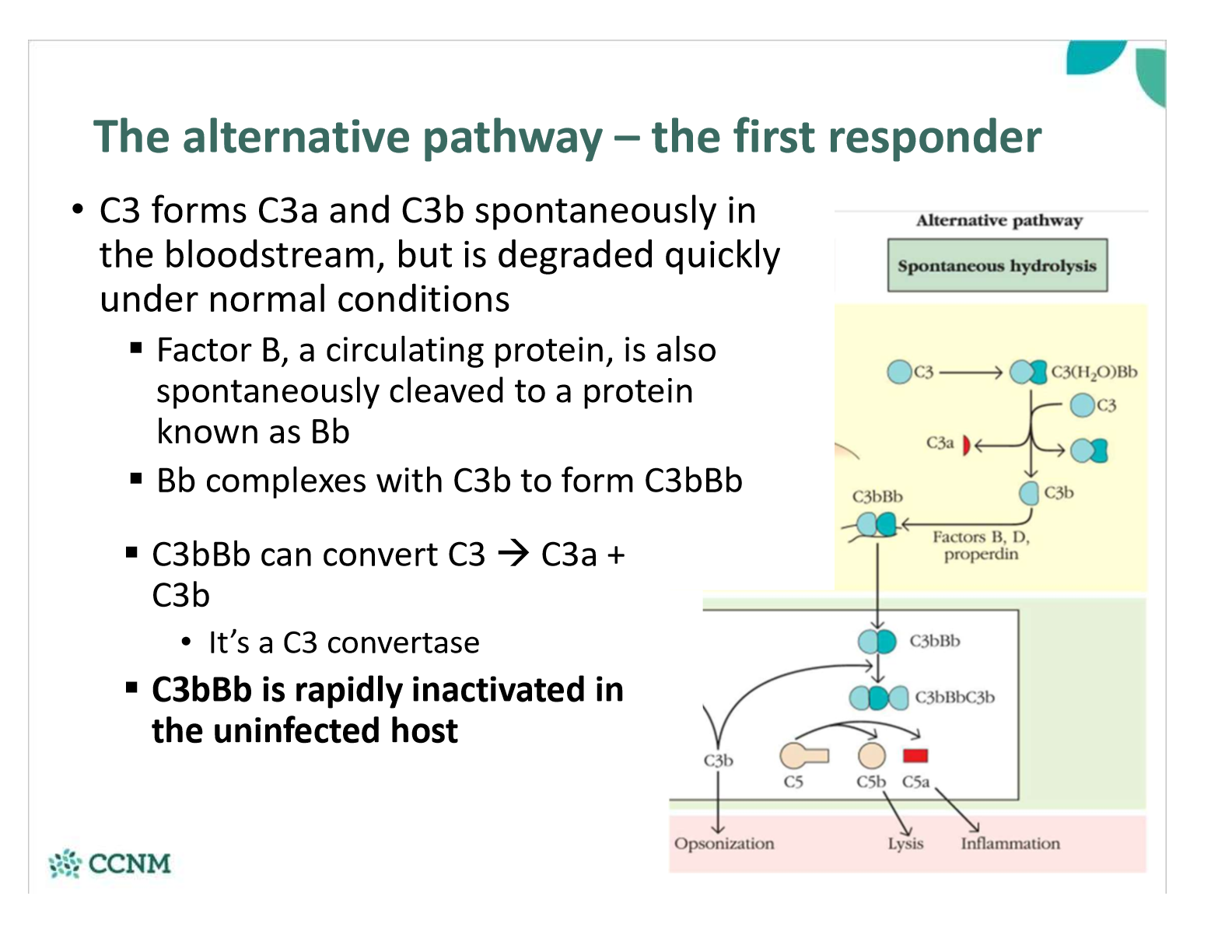
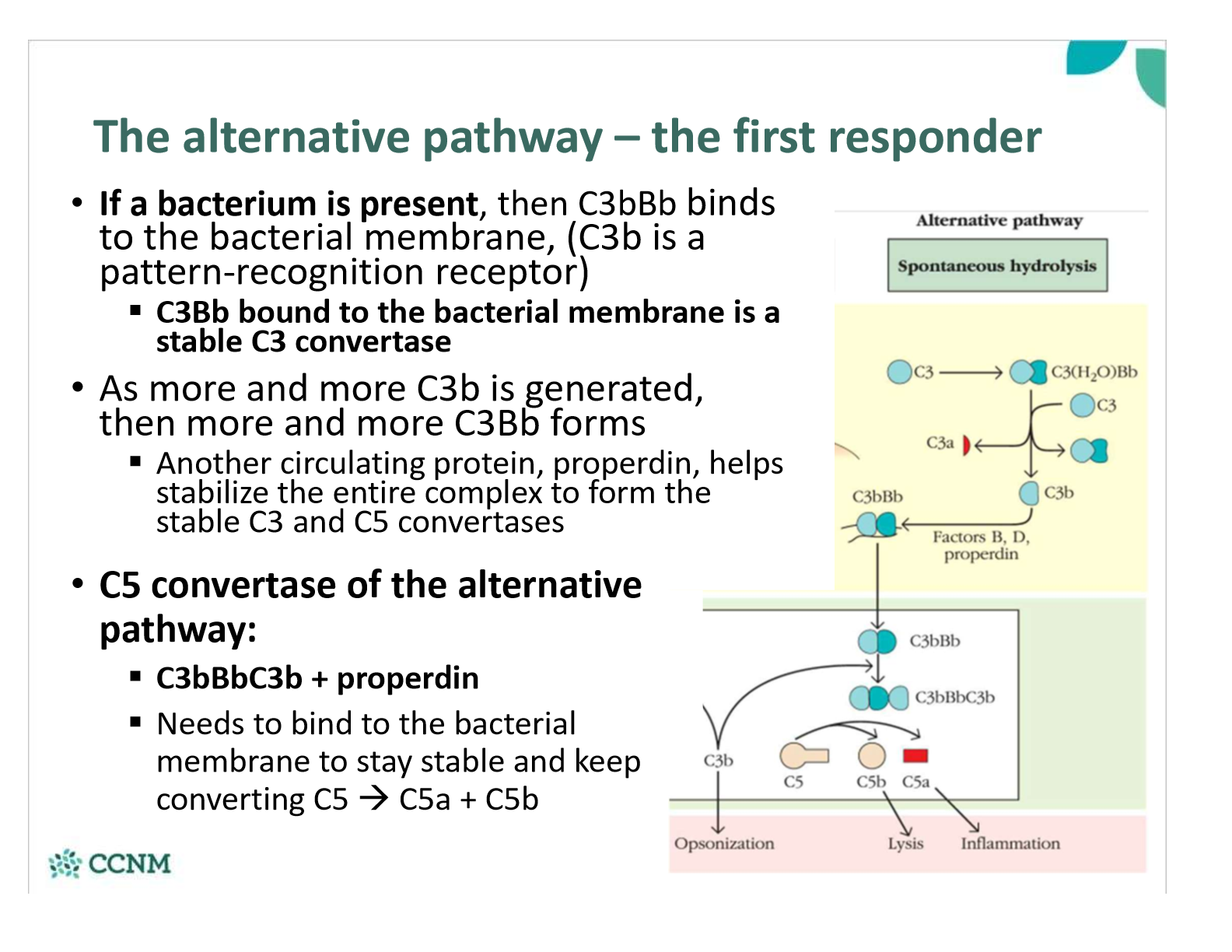
what happens in the alternative pathway if bacterium is present?
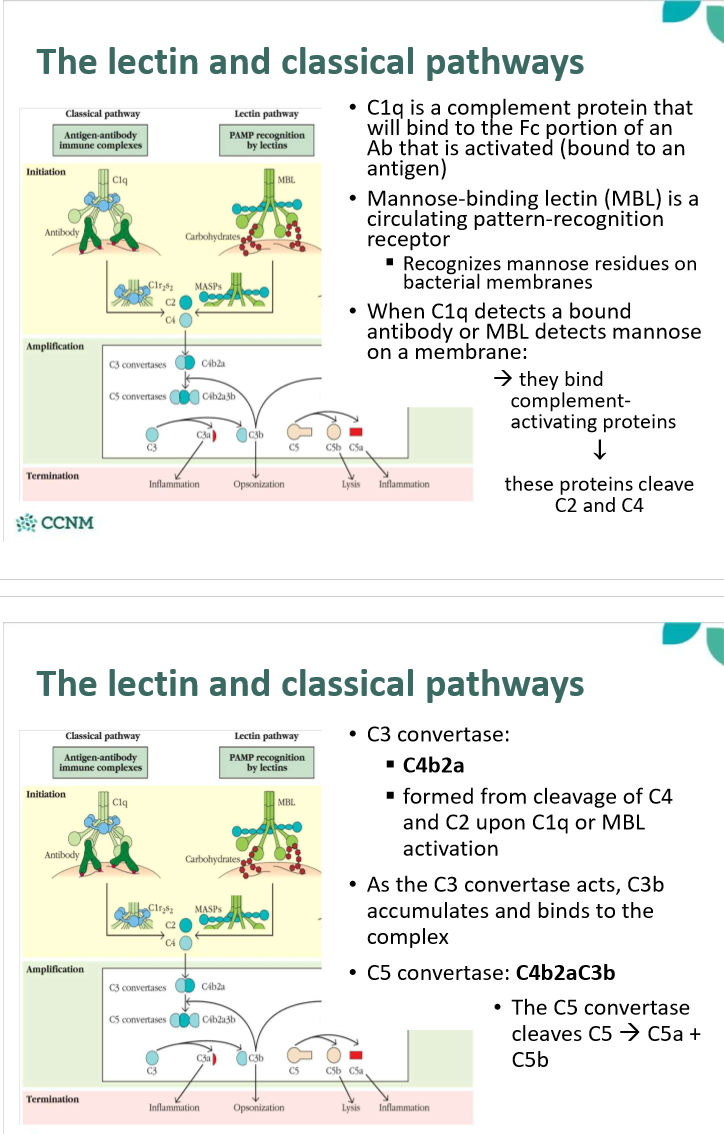
what happens in the lectin and classicial pathways?
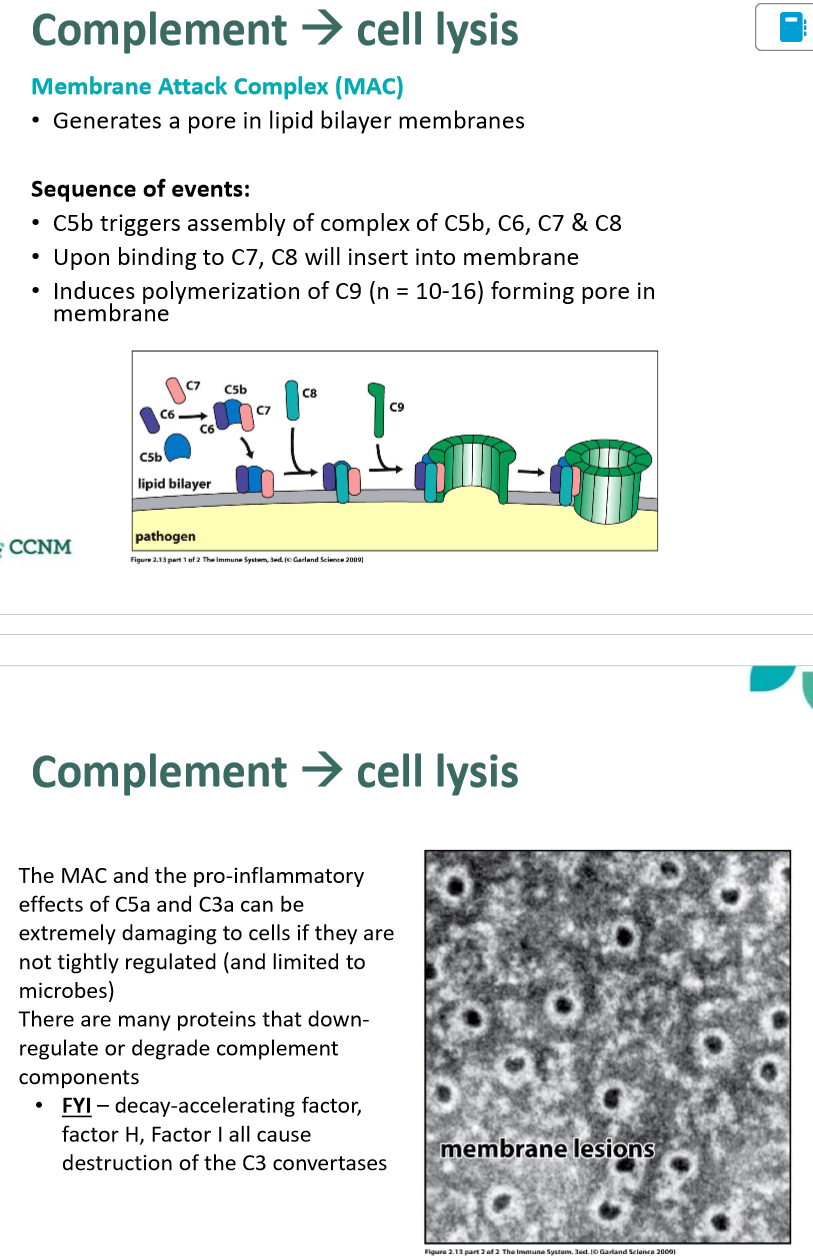
describe the role of the membrane attack complex (MAC)
What else can C3a and C5a do?
§They are both cause vasodilation, increased vascular permeability, smooth muscle contraction (i.e. bronchoconstriction) and histamine release from mast cells
§C5a is a chemotactic agent for a wide variety of cells (neutrophils & macrophages in particular)
Can people have a deficiency of complement?
§Yes – it’s a rare cause of immunodeficiency
§Inadequate complement proteins (C2, C3, C4, C5, MBL, MAC complex) tend to make patients vulnerable to bacterial infection
§Deficiencies in C1q highly predispose patients to systemic lupus erythematosus (discussed later in immunology)
•Photosensitivity rash, arthritis of small joints, renal failure, neurological vasculitis, neuropathies, and diverse effects on heart and lungs
•Prevalence: 50 – 100/100,000
§C1q helps macrophages to clear apoptotic bodies as well as initiate the classical pathway (C1q recognizes phosphatidylserine)
•Thought that continual presence of self-antigens (especially nuclear material in the extracellular space) from dying cells increases the likelihood of developing autoimmunity
•C1q may also have complex immunomodulatory roles with Th cells
Why is the alternative pathway the first responder, and the lectin/classical pathways more effective later?
§C3 is always present in the bloodstream – it’s constantly being produced by the liver, cleaved, and degraded
•If a microbe is present, C3b instantly binds (non-specifically) to the cell wall/membrane – if properdin and Bb also bind, then the stable C3 convertase forms very quickly
§Mannose-binding lectin does not circulate in high concentrations unless it is secreted by the liver in response to pro-inflammatory signals
§Significant quantities of antibodies take days – weeks to produce
where do TLRs recognize PAMPs?
•Toll-like receptors tend to recognize PAMPs in the ECF or in endosomes (not in the cytosol)
what do TLR1s detect?
can detect mycobacteria (like TB) and gram-negative bacteria
what do TLR2s detect?
can detect peptidoglycans – major component of cell wall of gram-positive bacteria
what do TLR3s detect?
double-stranded RNA à only found in viruses
what do TLR4s detect?
lipopolysaccharide (LPS), major component of gram negative bacteria
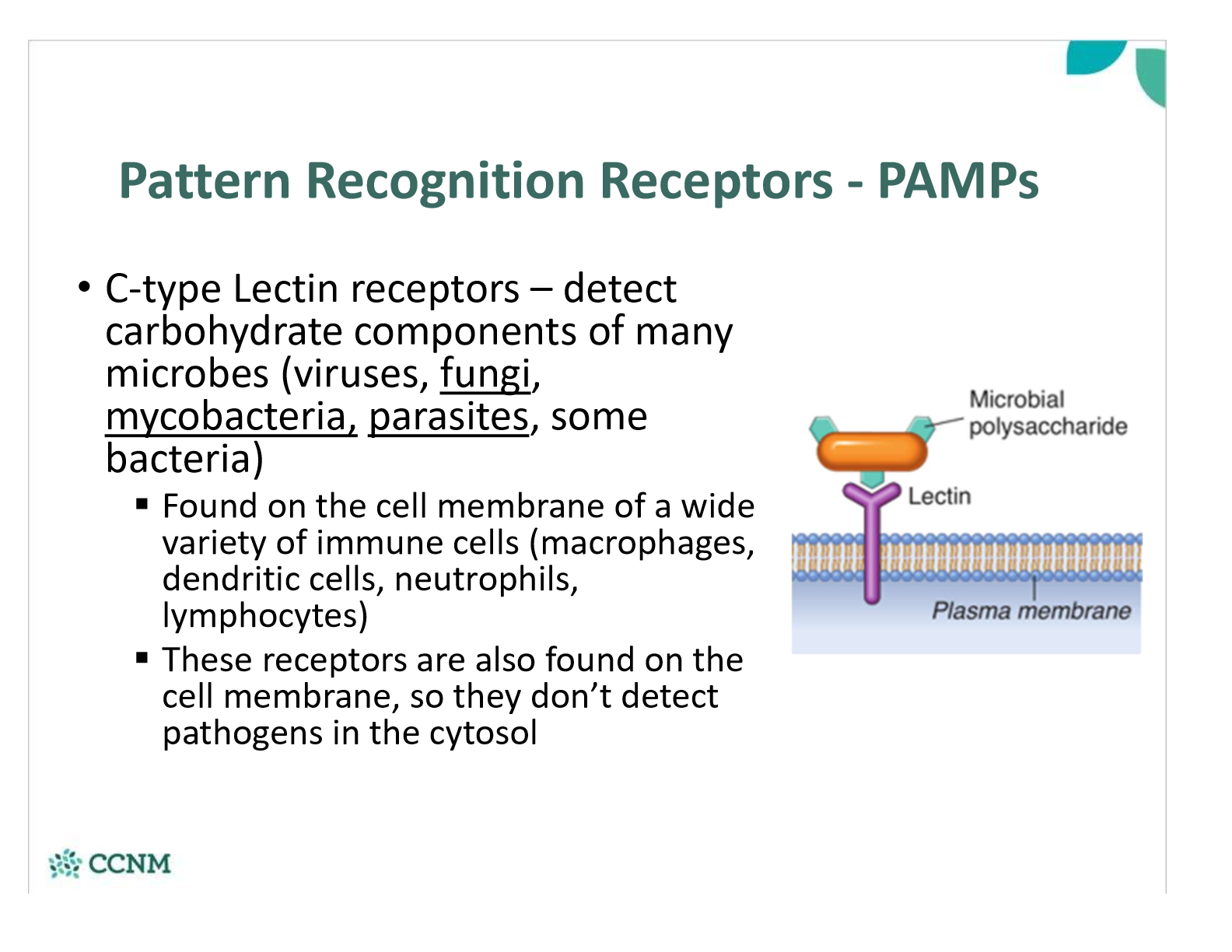
where are C-type lectin receptors found? what do they detect?
where are NOD-like receptors found? what do they detect?
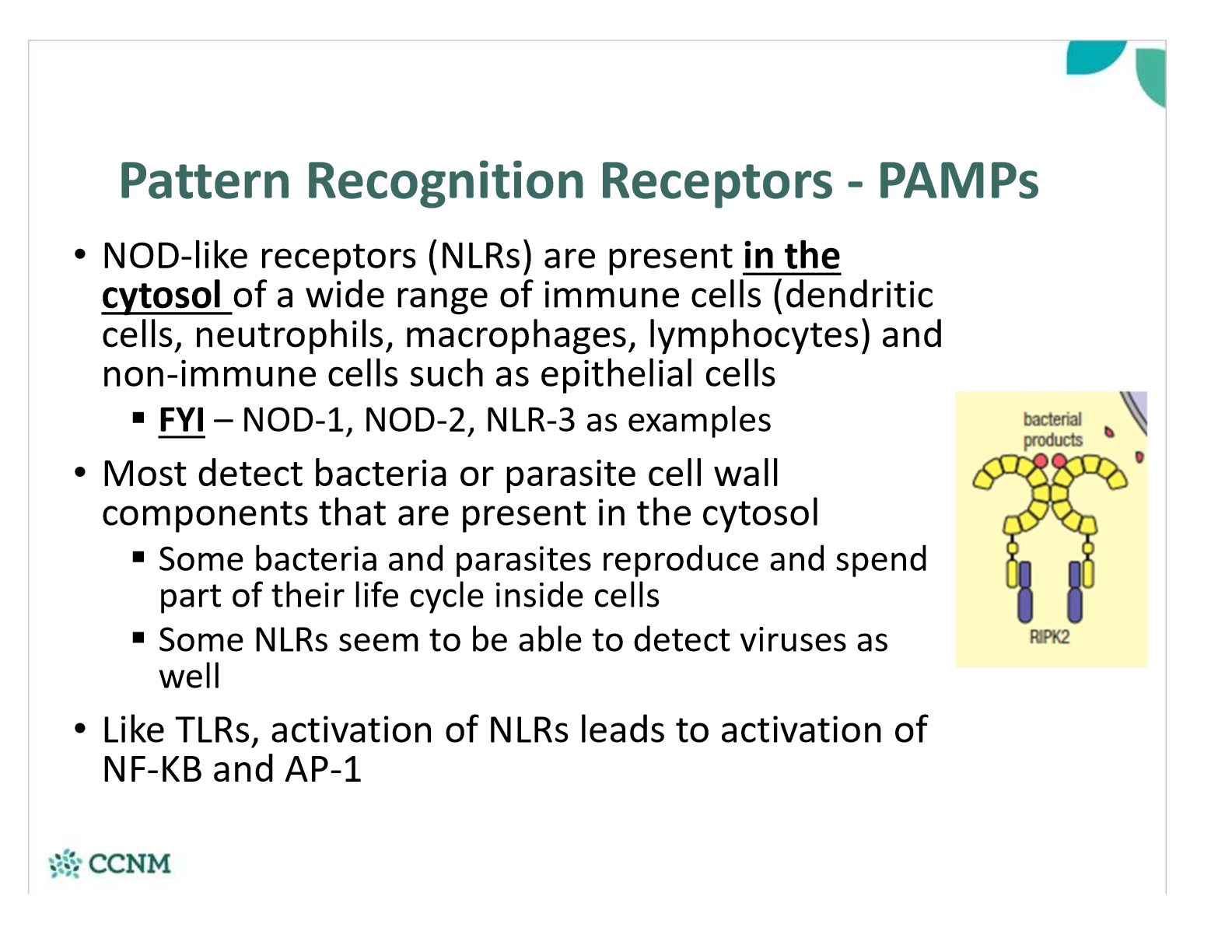
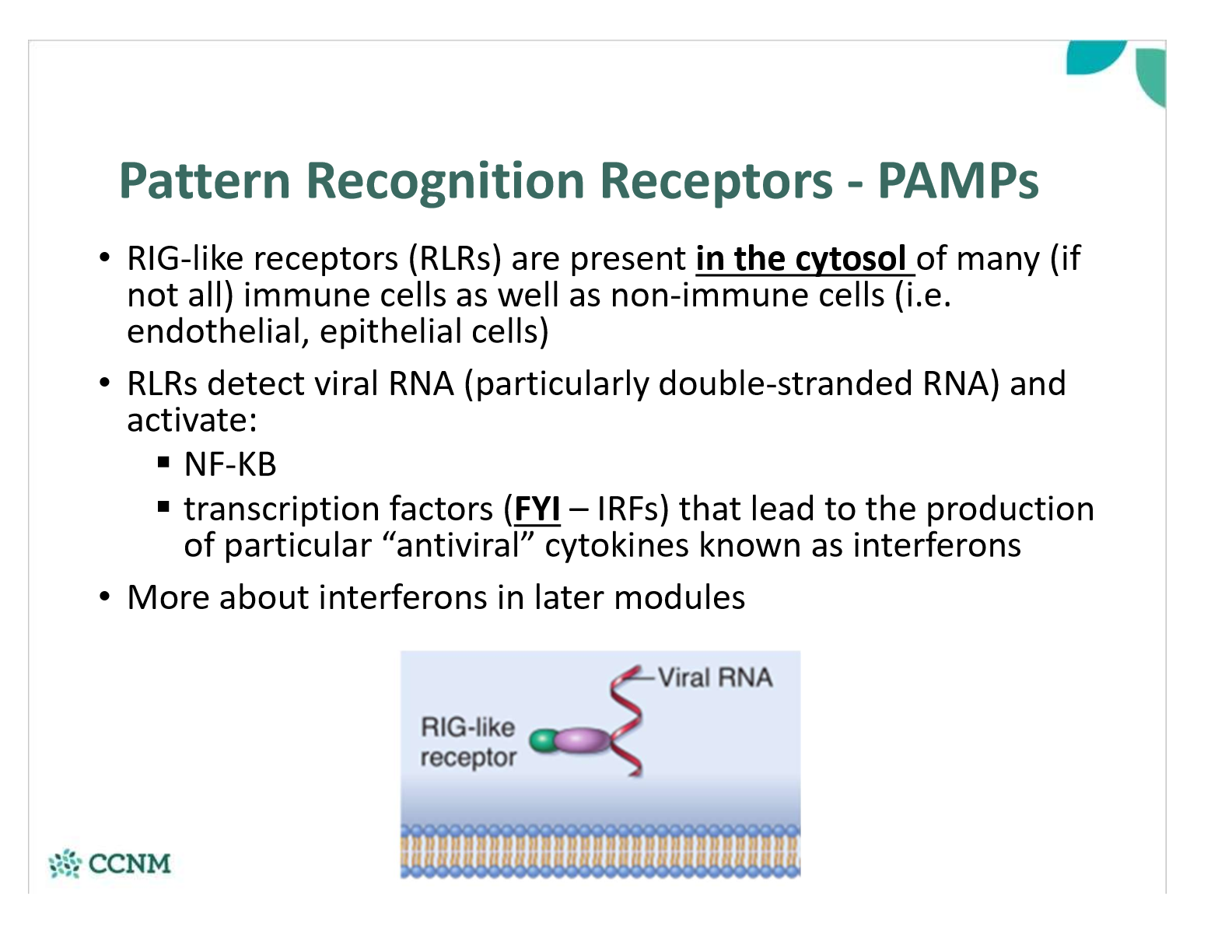
where are RIG-like receptors (RLRs) found? what do they detect?
what are examples of DAMPs?
how are DAMPs detected? what is the consequence?
describe the actions of an inflammasome
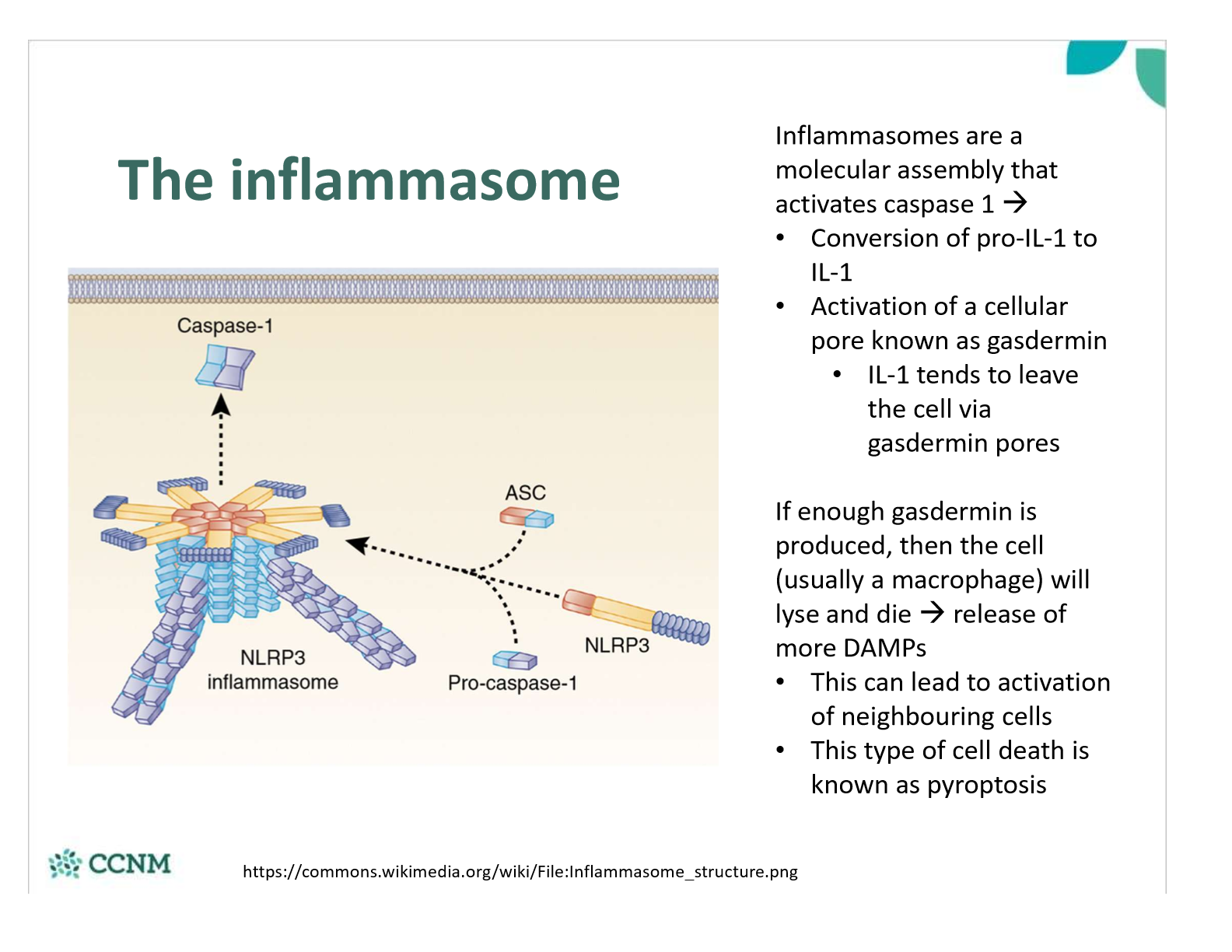
what is IL-1-beta? how is it generated? what are the consequences?
explain how resident macriphages are important in detecting PAMPs and DAMPs
explain how endothelila cells are important in detecting PAMPs and DAMPs
explain how epithelial cells are important in detecting PAMPs and DAMPs

list the major pro-inflammatory cytokines
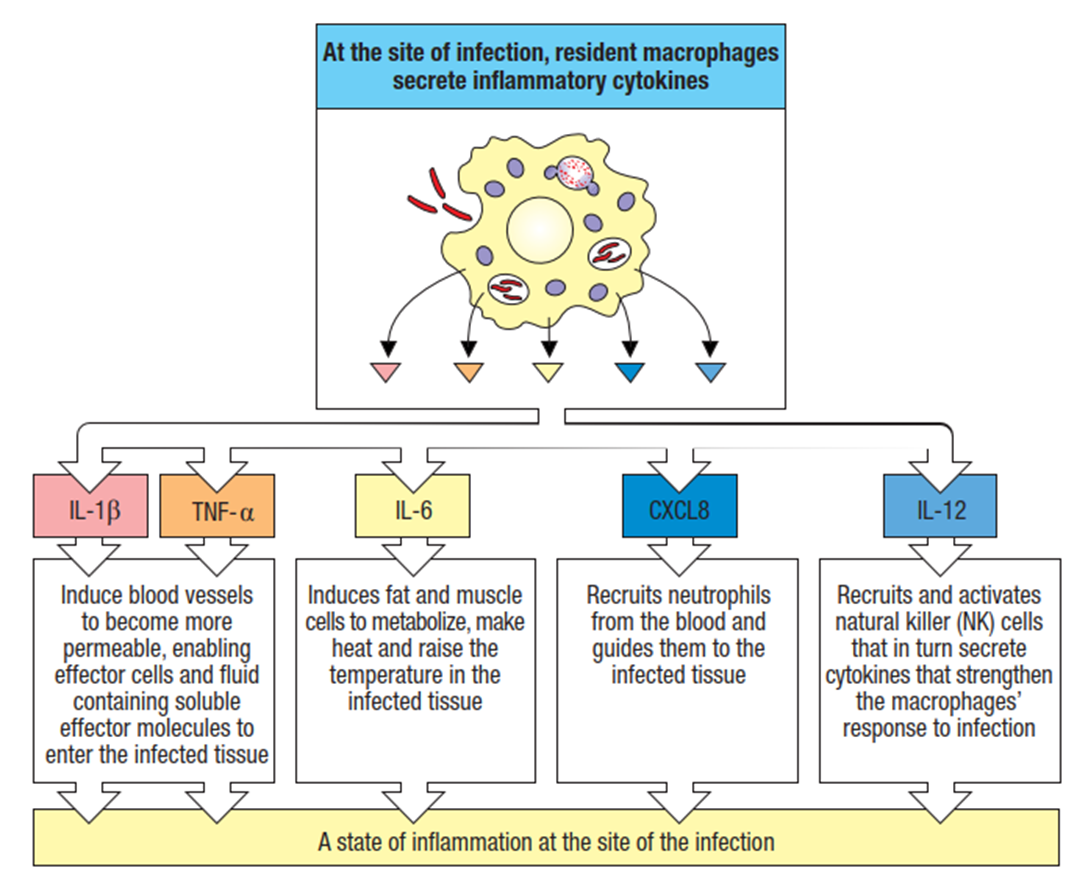
what are the sources of IL-1?
§Macrophages/monocytes, dendritic cells, keratinocytes, epithelial cells, endothelial cells
what are the sources of TNF-alpha?
§Macrophages/monocytes, dendritic cells, mast cells, NK cells, epithelial cells
what are the sources of IL-6>
§Macrophages/monocytes, dendritic cells, NK cells, epithelial cells, endothelial cells
explain how the functions of IL-1, TNF-a and IL-6 are redundant and pleiotropic
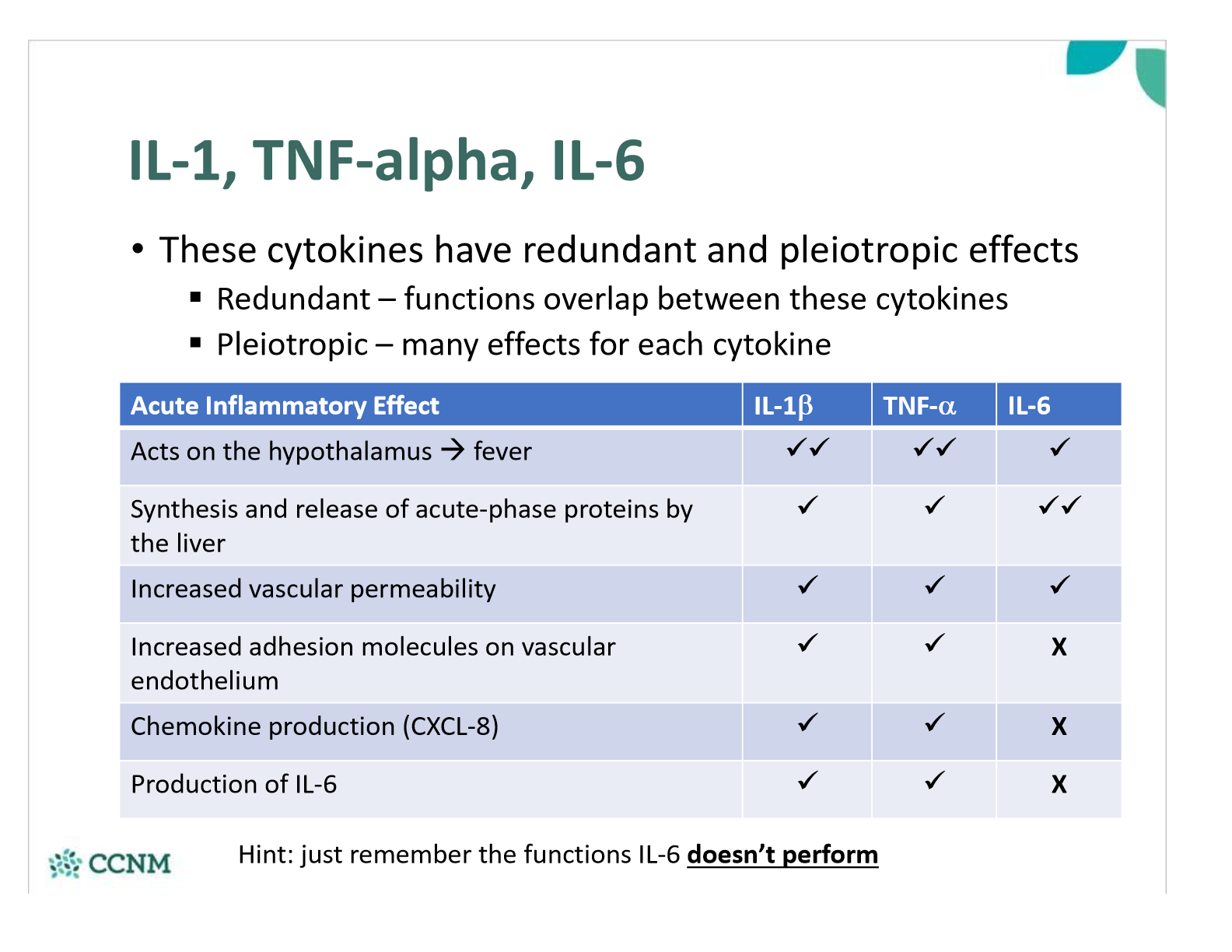
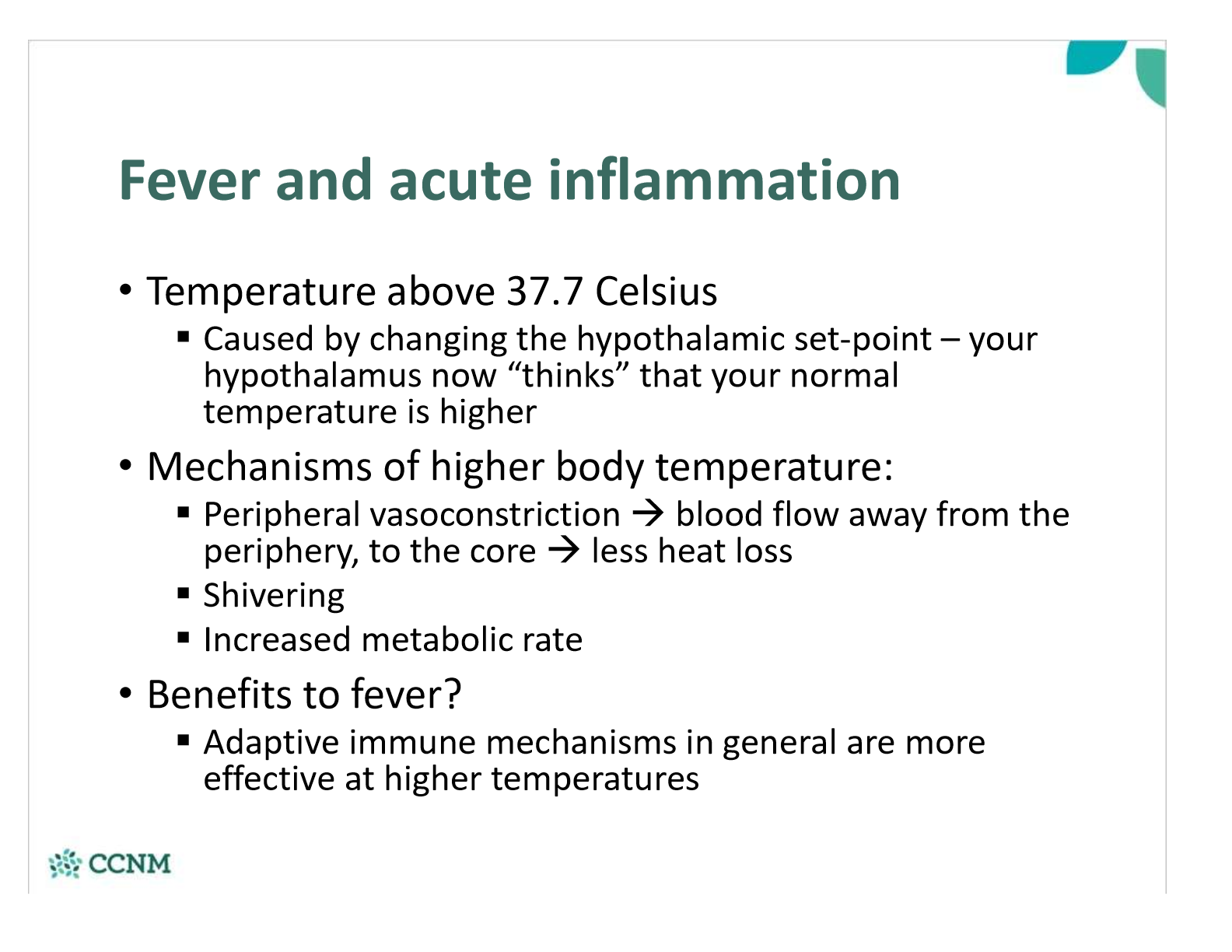
what are the systemic effects of a fever? are there any benefits?
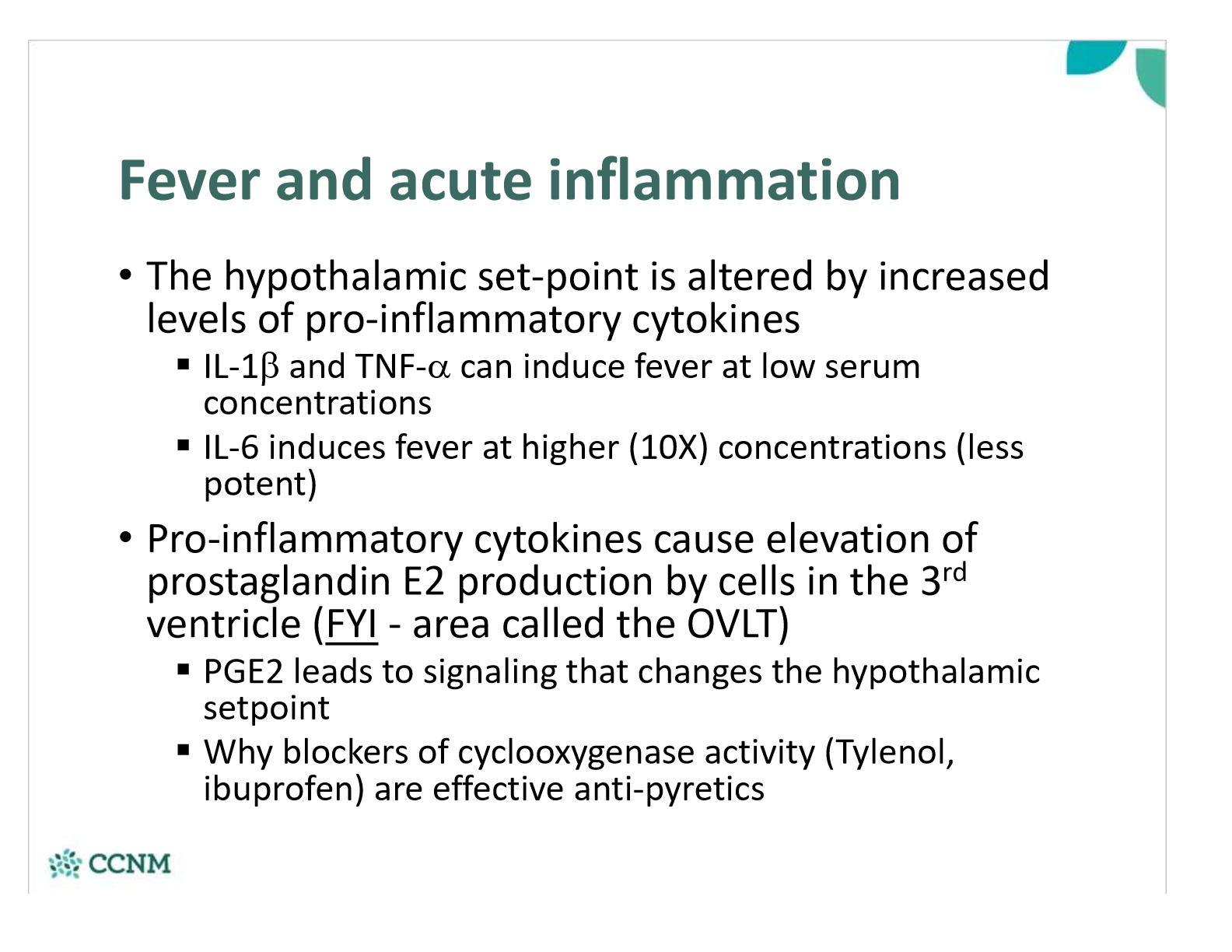
how are IL-1, TNF-a and IL-6 involved in fevers?
what are acute phase proteins
•Elevated levels of inflammatory cytokines – IL-6 in particular - cause the liver to increase the secretion of useful (from an acute inflammation perspective) proteins into the bloodstream
§Known as acute phase proteins
what is C-reactive protein?
C-reactive protein (CRP)
•Opsonin that binds to phosphorylcholine – a component of bacterial cell walls
•It can also activate C1q, and thus trigger the classical complement cascade when it binds to phosphorylcholine
•CRP is a common lab measurement ordered to diagnose inflammatory disease
what is ferrtin?
acute phase protein
•Binds to serum iron with high affinity – many microbes depend on iron for their metabolism, and ferritin sequesters it from these microbes
what is hepcidin
acute phase protein
•Interferes with intestinal transport of iron into the bloodstream – this also sequesters iron from microbes
what is Mannose-binding lectin (MBL)
acute phase protein
PRR that initiates the lectin complement cascade
what is Serum amyloid protein A (SAA)
acute phase protein\
•Modulates (usually increases) the activation of the inflammasome and TLRs
•Opsonizes some gram-negative bacteria
compare and contrast the two main groups of interferons
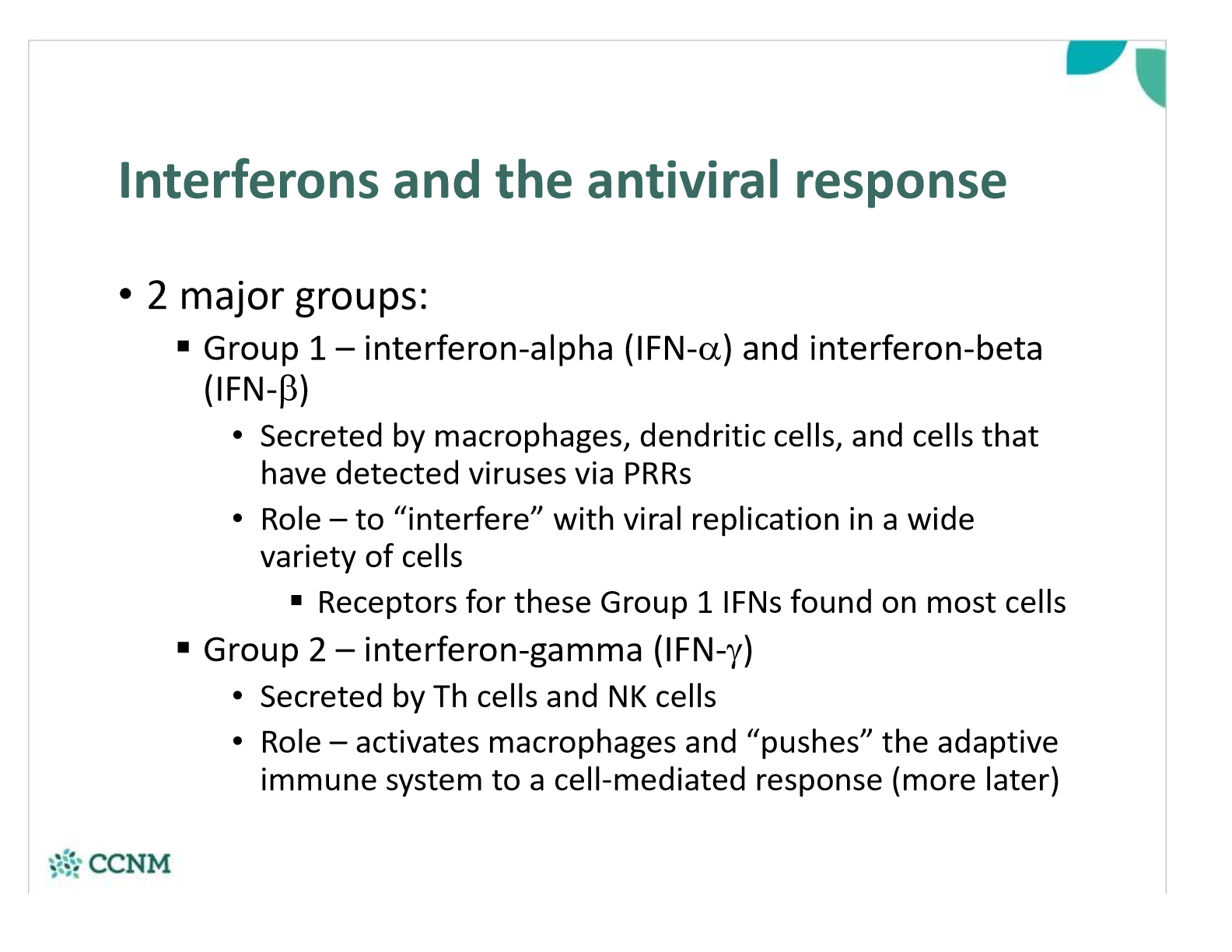
what are the signalling methods type 1 interferons use?
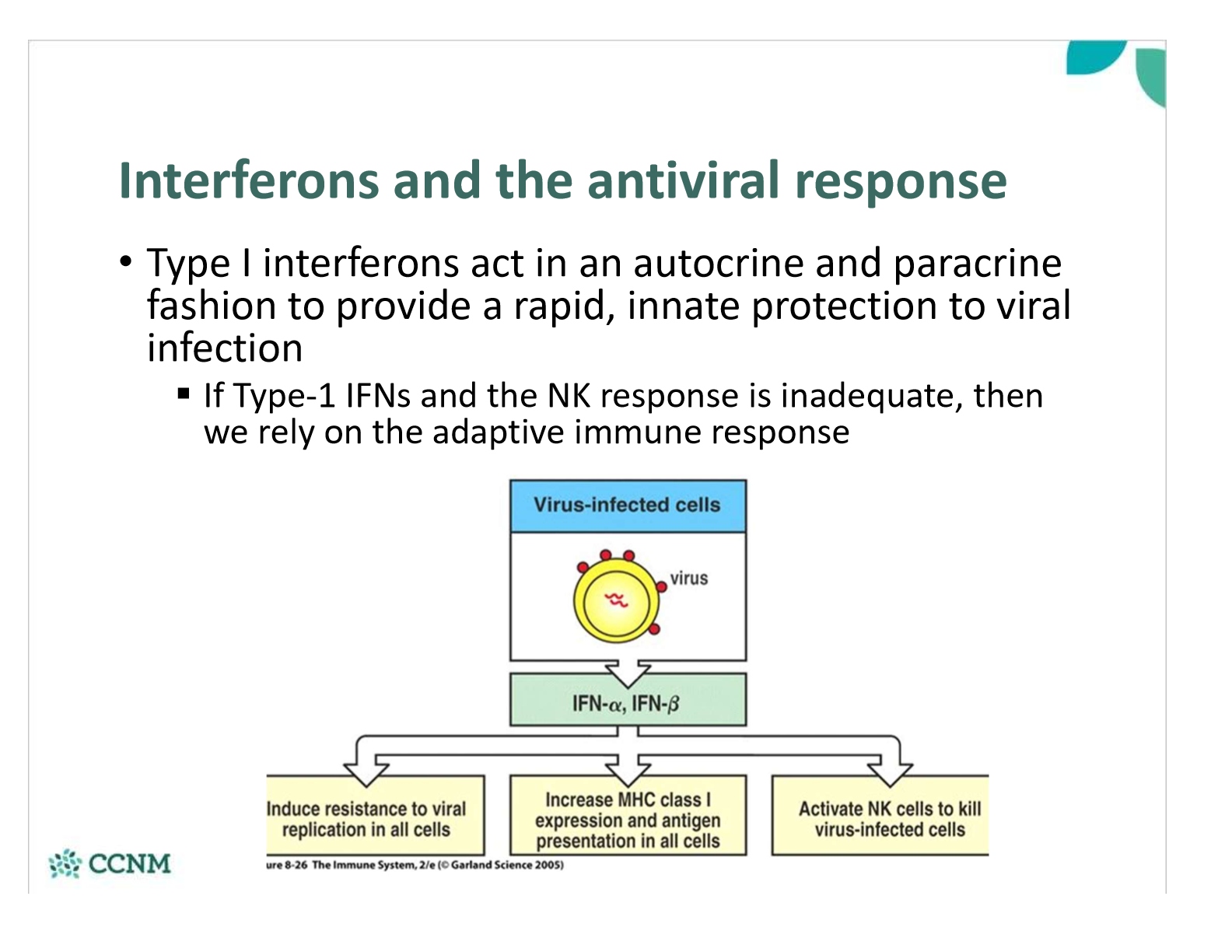
•How do interferons “interfere” with viral replication inside cells?
§Inhibit of protein translation in the presence of viral RNA
§Degrading viral mRNA
§Inhibition of viral protein assembly
•The net result is that type 1 IFNs reduce the ability of infected cells and the virus to synthesize and assemble proteins
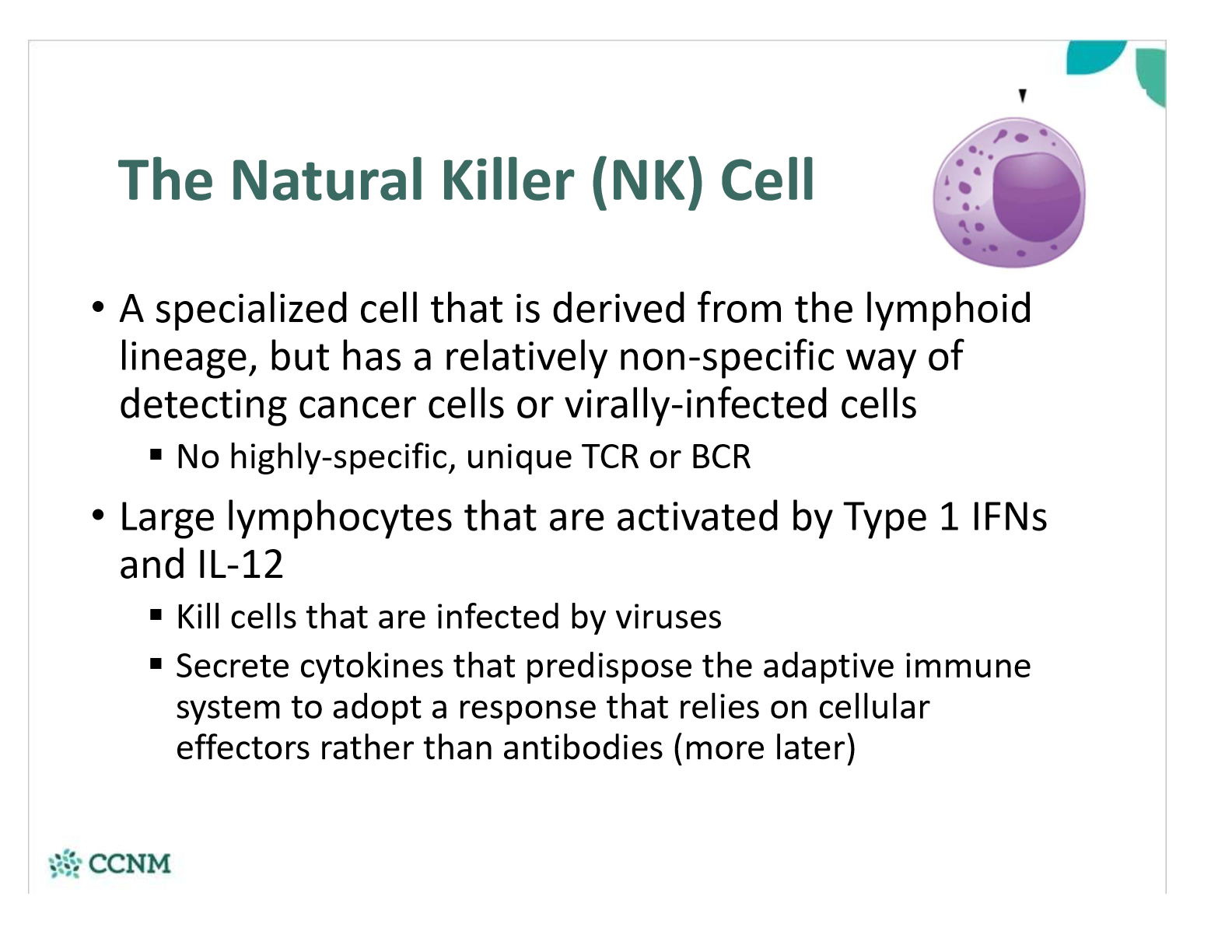
what is a NK cell? what do they do?
survey the body for infected or stressed abnormal cells (which greatly increases the presense of type 1 IFNs)
compare the two types of receptors on NK cells
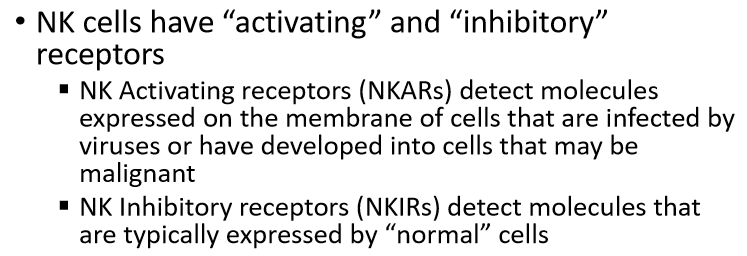
describe an examples of a NKAR

describe an examples of a NKIR

what needs to happen to an NK before it is activated?

what happens once an NK cell is activated and licensed?

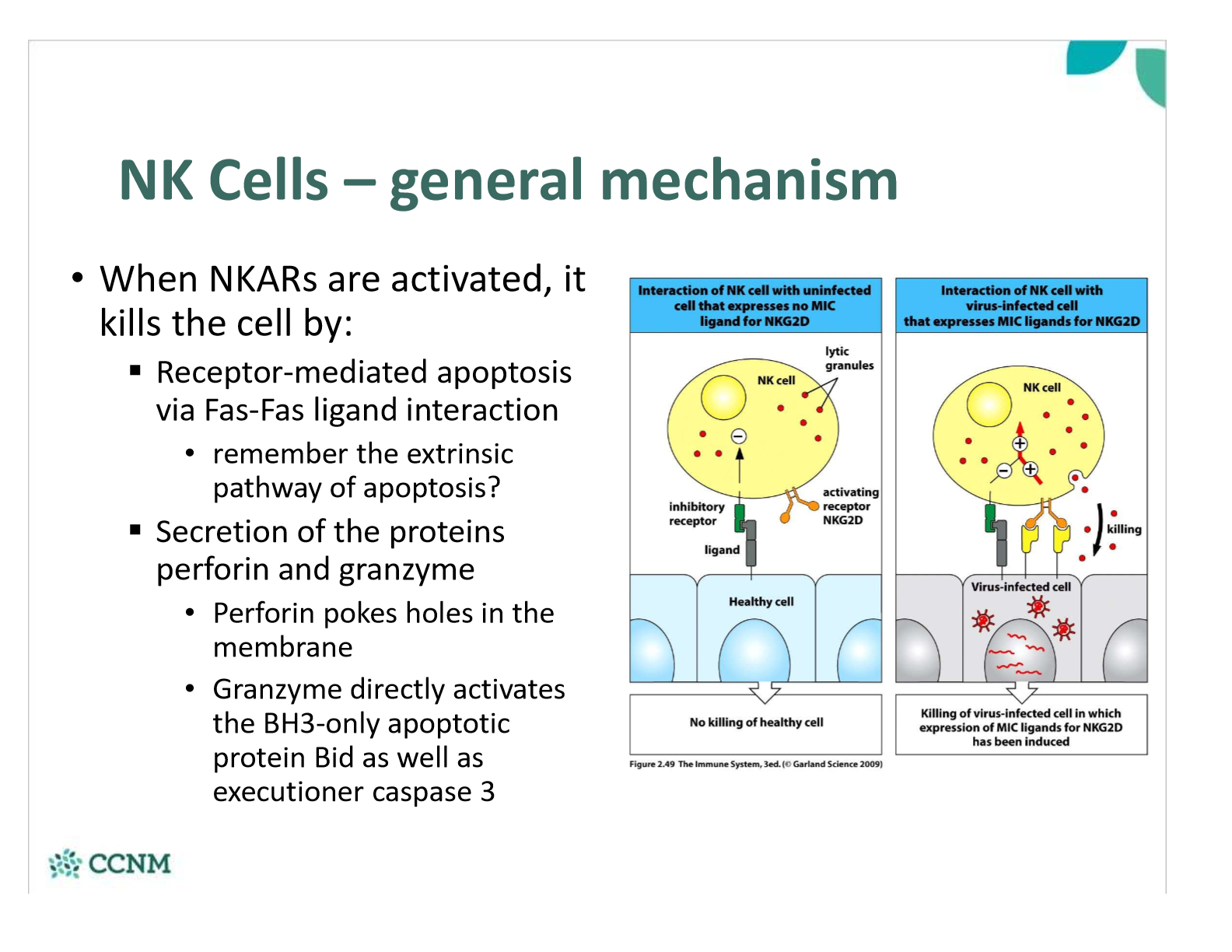
what mechanisms do NK cells use to kill cells?
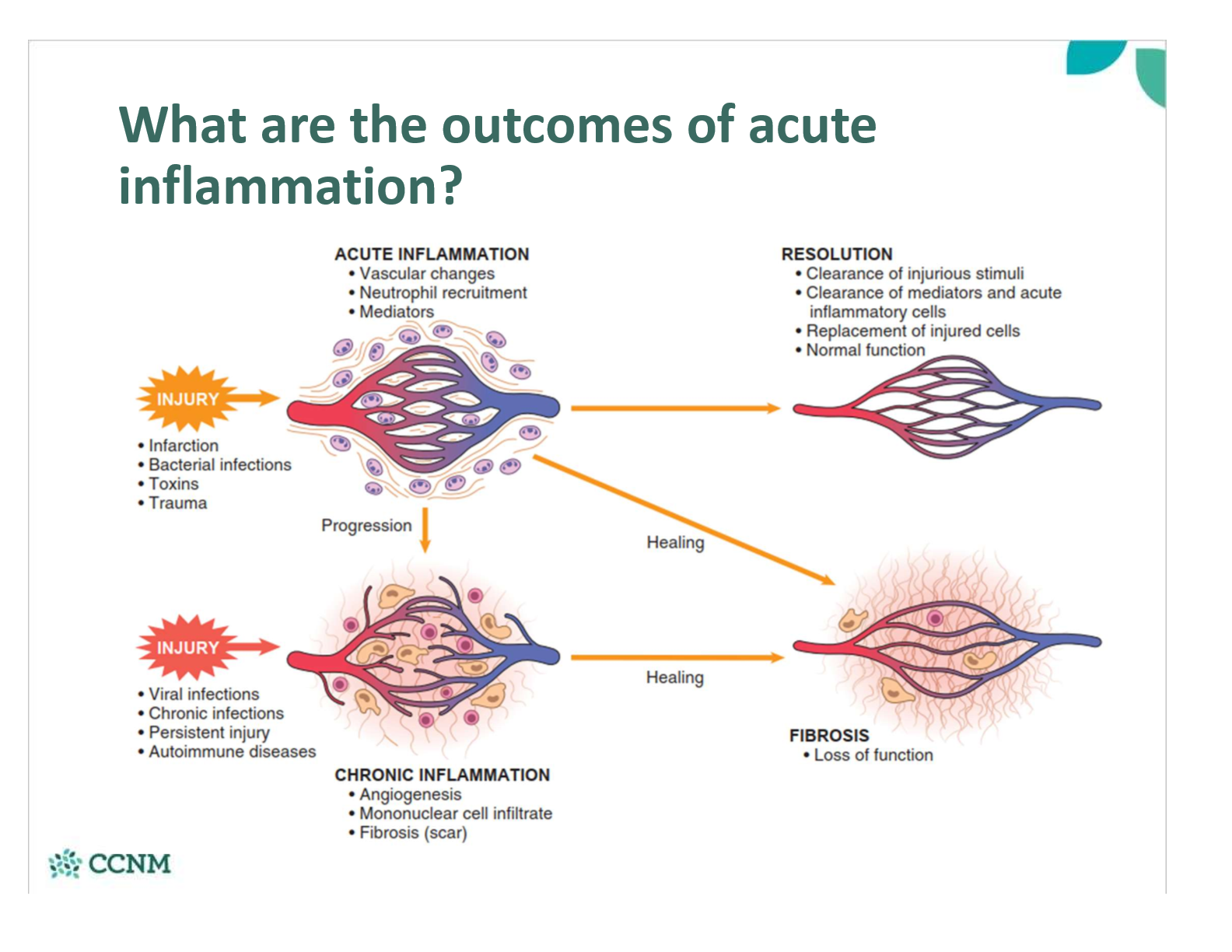
what are the outcomes of acute inflammation?