
Looks like no one added any tags here yet for you.
What is a solution?
A homogenous mixture of two or more components
Homogeneous – the same throughout (a mixture at the molecular level).
Solution Terminology
Solvent
• major component
Solute
• minor component
Most solutions – solid solute and liquid solvent
• Air (gas/gas)
• Bronze (Zn/Cu)
• Beer ethanol/water
What is the difference between a solution
and a mixture?
Solution:
• Homogeneous (same composition
all the way through the sample)
• Once solution is formed it (typically)
will not “unmix”
• Examples
• salt and water
• sugar and water
• sports drinks
Mixture:
• Heterogeneous – different
composition depending on sample
point
• Relatively easy to “unmix”
• Examples
• sand and salt
• granite
• salad dressing
• Pepto Bismol
Solution by Mass
You make up a solution of 100 g water and 5 grams NaCl.
What will be the resultant mass?
Exactly 105 grams
Solution by Volume
You make up a solution of 100 mL water and 5 mL of ethanol
(CH3CH2OH).
What will be the resultant volume?
Between 105 and 100 mL
Making a Solution:
Use a volumetric flask
Add the solute to the flask and make up to the required volume
Read the bottom of the meniscus
When you look at a liquid in a flask, the top of the liquid is not flat, it is curved. The curve is called the meniscus. You want the bottom of the curve to touch the line on the flask.
Why can’t we add 5.84 g NaCl to 100 mL of water? Because, the volumes are not additive.
Put 5.84 g NaCl in the flask and add enough water for the bottom of the liquid level to touch the line on the flask.
How do we represent a solution?
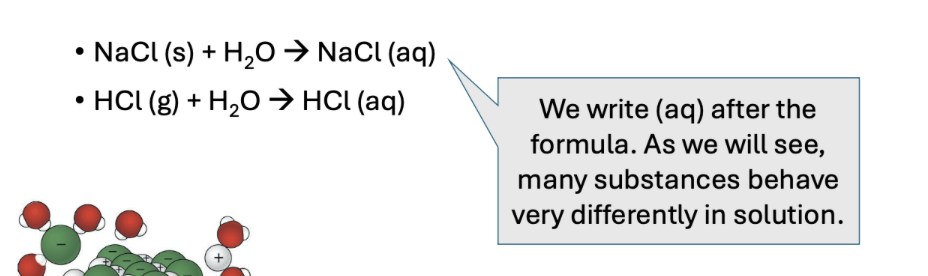
(aq) means aqueous – dissolved in water.
When you see (aq), it is a giveaway that you have a solution.
Remember there is no such thing as NaCl molecules, so it can be misleading to write NaCl (aq). You must think about what this means and how the NaCl would actually exist in solution.
Solutions (of liquids):
Immiscible:
The solute and solvent do
not mix up – may form two
layers – e.g. oil and water
Note: This is not all or
nothing!
Miscible:
• The solute and solvent mix
up (form a solution) – once
mixed they do not unmix
• A solution forms!
Solutions:
Saturated
• No more solute will dissolve
(how will you know that?)
Unsaturated
• If you add more solute it will
dissolve
Concentrations of Solutions - Units:
What units are commonly used?
• Molarity (mol/L)
• A 1 M solution contains 1 mol of solute dissolved to make 1 L of solution.
• Allows conversions between moles of solution, volume of solution and mass of solute.
Other units
• g/mL, ppm, pp
To know if a solution is saturated or unsaturated, you need to be able to keep track of how much solute is dissolved in the solution (solvent).
There are many units that was can use to describe the concentration of a solution. Ask for some concentration units.
The most common way is to use molarity.
You may have heard of other units: g/mL, ppm, ppb (for Pb in water); we will get to these in a few minutes

How big is a mole?
6. 022 x 10^23 of something
Represents a large quantity of anything!
Like a dozen of something!
Atoms, moles, molecules, ions, etc.
How many grams of NaCl (molar mass of NaCl 58.4 g/mol) are required to make 1.0 L of a 0.1 M solution?
5.84 g

Which solution is concentrated (and which is dilute)?
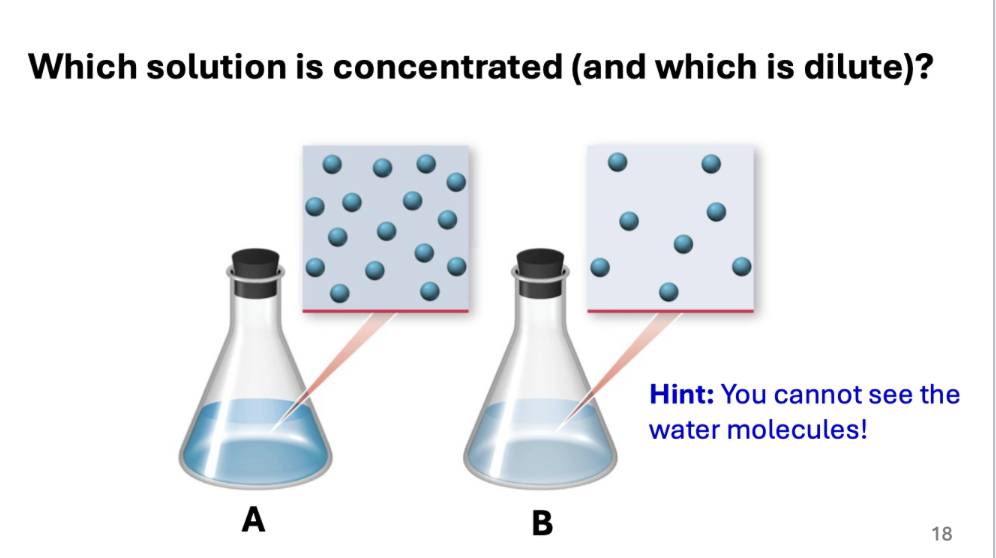
A
Dilution:
When 0.1 M HCl is diluted with water, what changes?
volume
concentration
What stays the same?
mols of HCL
mols of solute
Dilution means water is added to lower the concentration
when we make solutions, we start with a concentrated solution and then dilute it.
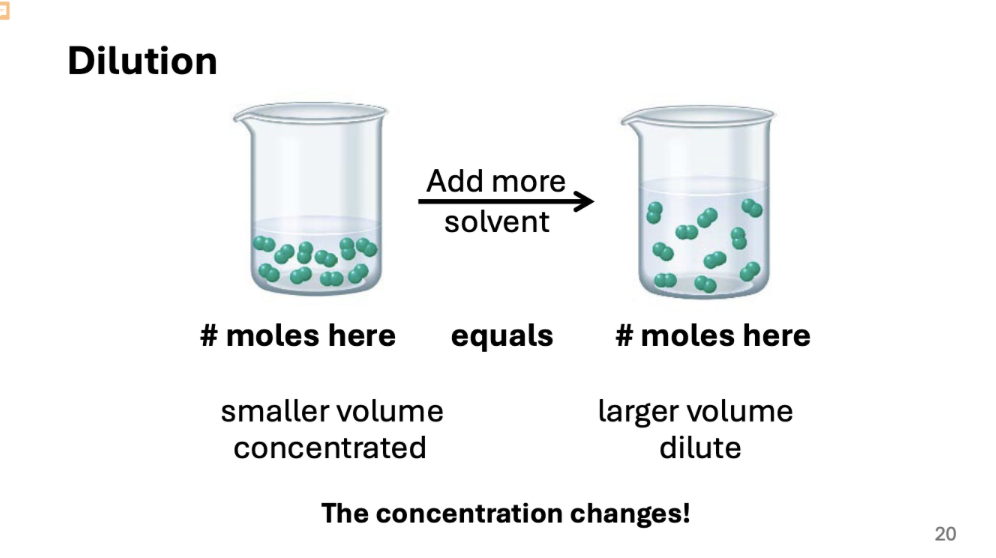
Diluting Solutions:
If you add water to a concentrated solution what stays constant?
• The number of moles of solute!
moles solute in concentrated solution = moles solute in dilute solution
Remember: moles solute = M (mol/L) x V (L)
• So, if the moles of solute stay the same during dilution, then for the
solutions: M1V1=M2V2
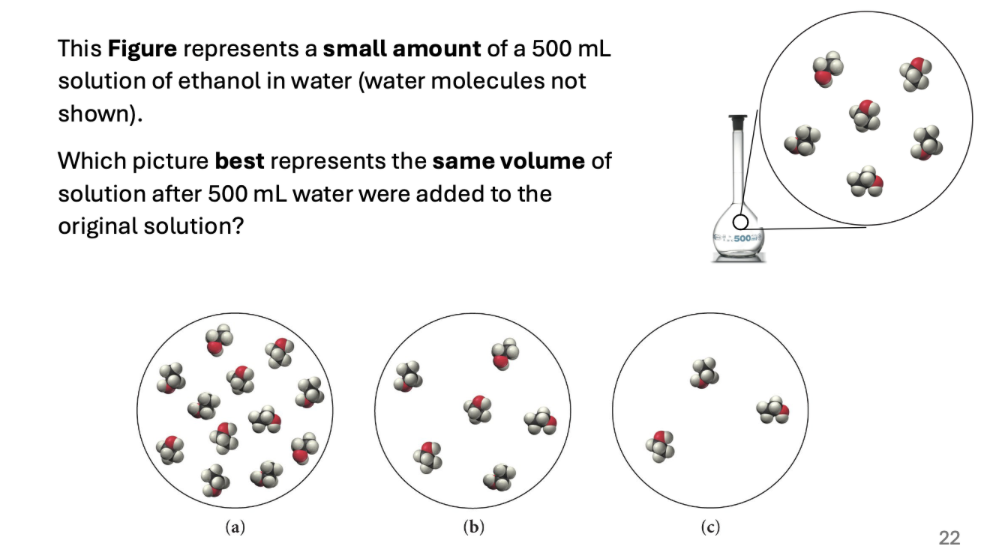
B
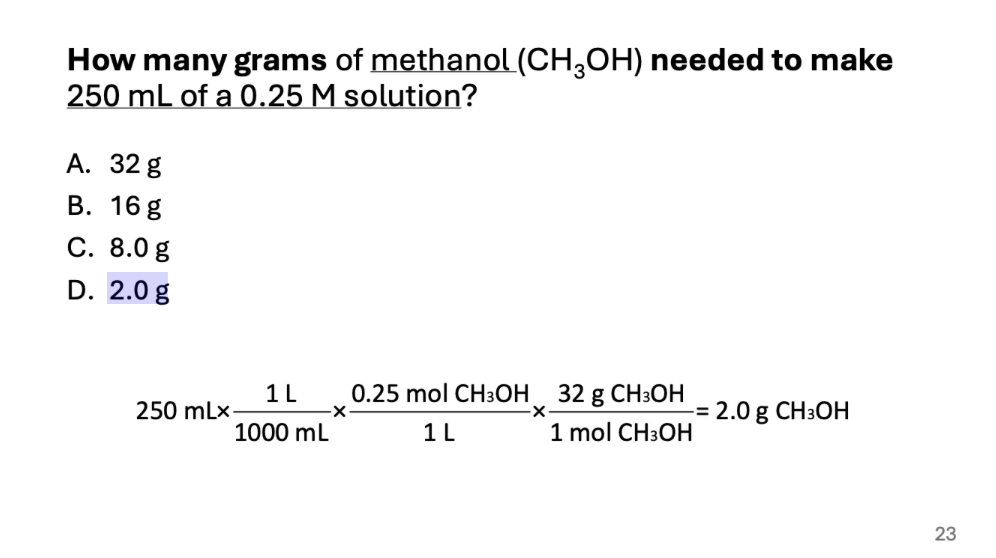
How many grams of methanol (CH3OH) needed to make 250 mL of a 0.25 M solution?
2.0 g
How much 0.25M methanol (CH 3OH) would be needed to make 1 L of a 0.10 M solution?
0.40 L
𝑀1 𝑉1 = 𝑀2 𝑉2
Concentration by Mass or Volume:
• For example: g/L or mg/L
• Easier to prepare
For a 2 g/L solution – add 2 g solute and make up to 1L solution
For a 2 mL/L solution – add 2 mL solute and make up to 1L solution
25
- Another way to prepare solutions is by mass or volume. These solutions are easy to prepare without many calculations.
- You would still use a volumetric flask to measure the volume of the solution.