Organic Chemistry Conformations, Nomenclature, and Thermodynamics
1/23
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
24 Terms
What are Newman projections used for?
To visualize the conformation of molecules by looking down a bond.
What is the lowest energy conformation of 2,2,4-trimethylpentane?
The conformation with the largest groups in equatorial positions.
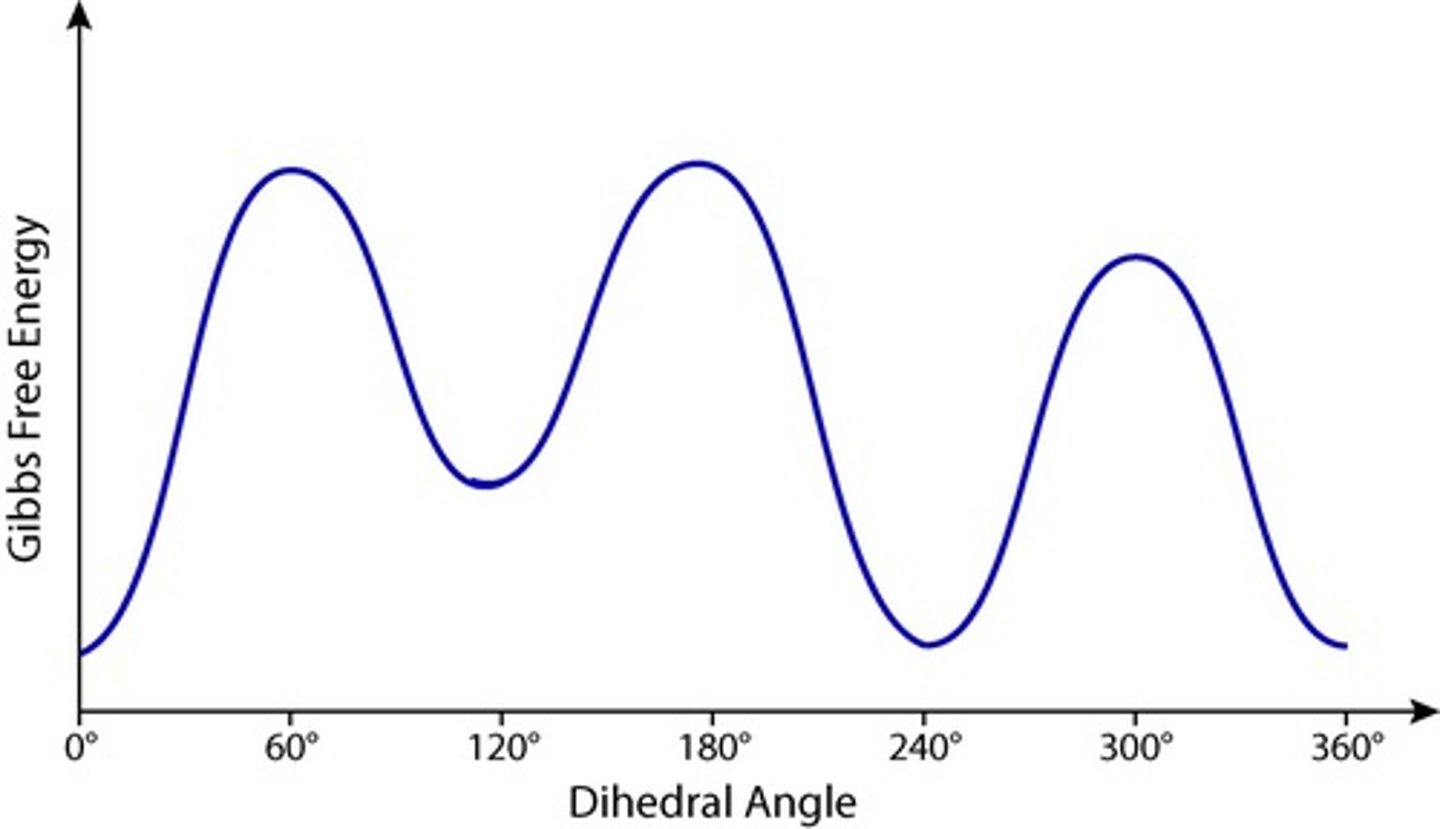
What is the significance of the energy diagram in conformational analysis?
It indicates the relative energies of various conformers.
What is the preferred conformation for 1-ethyl-1-methylcyclohexane?
The conformation where the ethyl group is in an equatorial position.
What is the cis/trans nomenclature used for?
To describe the spatial arrangement of substituents on cyclic compounds.
What is the energetically favored conformation for cis-1,3-dimethylcyclohexane?
The conformation where both methyl groups are in equatorial positions.
What happens to the concentration of CH3OH when temperature increases in a reaction converting three moles of gas to one?
The concentration of CH3OH decreases.
What does a negative DGrxn indicate about a reaction?
The reaction is thermodynamically favored.
What does K > 1 imply about a reaction at equilibrium?
There are more products than reactants.
What is the equilibrium expression for the reaction 2 SO3(g) ⇌ 2 SO2(g) + O2(g)?
K = [SO2]^2[O2] / [SO3]^2.
What does a small equilibrium constant (K < 1) suggest about the concentrations of reactants and products?
The concentration of reactants is higher than that of products.
What is the relationship between DGrxn and DHrxn when K < 1?
DGrxn is positive, indicating the reaction is enthalpically unfavored.
What is the effect of increasing temperature on a reaction that is entropically favored?
DGrxn decreases and K increases.
What is the preferred conformation for trans-1-bromo-4-methylcyclohexane?
The conformation where both groups are equatorial.
What is the predicted sign of DSrxn for a reaction that forms fewer moles of gas?
DSrxn is negative.
What does the term '1,3-diaxial interactions' refer to?
Steric strain between axial substituents on a cyclohexane ring.
What is the significance of the flask observations before and at equilibrium?
They indicate the direction and favorability of the reaction.
What is the effect of steric bulk on chair conformations?
Larger groups prefer equatorial positions to minimize steric strain.
What is the equilibrium expression for the reaction CO(g) + 2 H2(g) ⇌ CH3OH(g)?
K = [CH3OH] / ([CO][H2]^2).
What is the expected concentration of CH4 if K = 0.020 and [C2H2] = 0.010 M, [H2] = 0.030 M?
[CH4] = 0.0037 M.
What does a negative DSrxn indicate about the dispersal of energy in products vs. reactants?
Energy is less dispersed in products than in reactants.
What is the impact of steric interactions on the stability of chair conformations?
Increased steric interactions lead to higher energy conformations.
What is the relationship between enthalpy and entropy in determining reaction favorability?
Both must be considered to predict the overall favorability of a reaction.
What is the effect of increasing temperature on an entropically unfavored reaction?
It makes the reaction less thermodynamically favored.