solids, liquids and gases
1/18
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
19 Terms
What is density =
Mass/volume (in kg/m³)
What is density definition
The mass per unit volume of a material
Practical : determining density - regular objects
Place the object on a digital balance and note down its mass
Use either the ruler, Vernier callipers or micrometer to measure the object’s dimensions (width, height, length, radius) – the apparatus will depend on the size of the object
Repeat these measurements and take an average of these readings before calculating the density
Practical : determining density - irregular shapes
Place the object on a digital balance and note down its mass
Fill the eureka can with water up to a point just below the spout
Place an empty measuring cylinder below its spout
Carefully lower the object into the eureka can
Measure the volume of the displaced water in the measuring cylinder
Repeat these measurements and take an average before calculating the density
Measuring the density of liquids
Place an empty measuring cylinder on a digital balance and note down the mass
Fill the cylinder with the liquid and note down the volume
Note down the new reading on the digital balance
Repeat these measurements and take an average before calculating the density
What is pressure definition
The concentration of a force or the force per unit area
Pressure (Pa) =
Force (N)/ area (m²)
The pressure at a point in a gas or liquid at rest acts …
Equally in all directions
Pressure difference = (in liquid)
Height x density x gravitational potential energy
Shape of solids, liquids, and gases
Solids :
A definite shape (they are rigid)
A definite volume
Liquids
No definite shape – they are able to flow and will take the shape of a container
A definite volume
Gases
No definite shape – they will take the shape of their container
No fixed volume – if placed in an evacuated container they will expand to fill the container
highly compressible bcs they have large gaps between particles which means it is easier to push then together
Changes of state
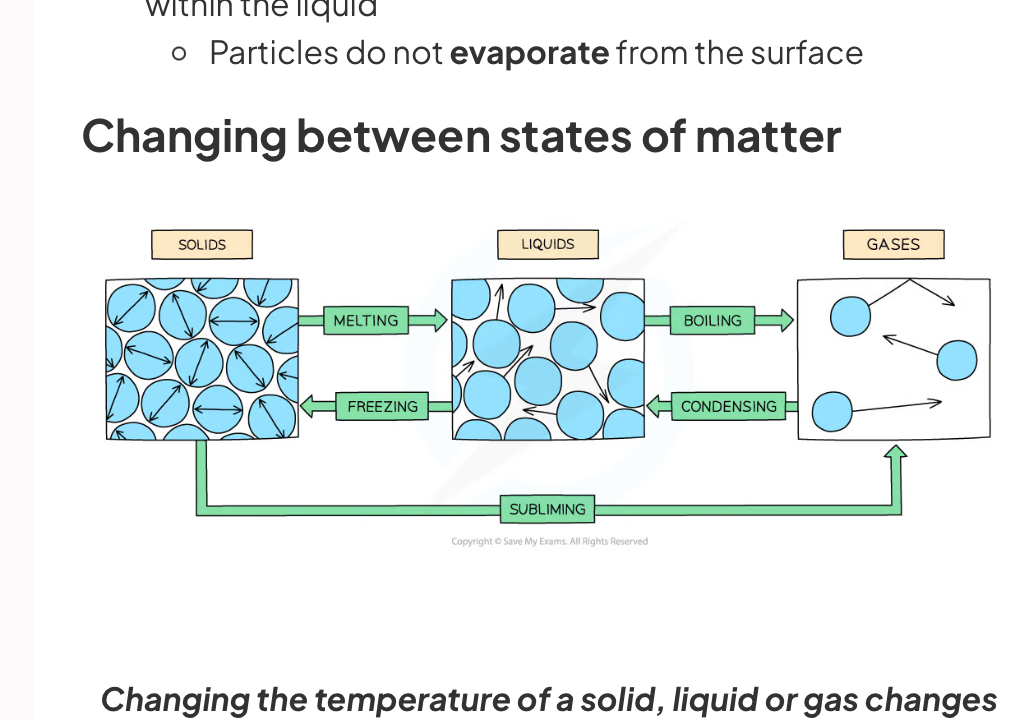
What happens when a solid is heated and melts
Thermal energy transfer takes place and supplies the particles in the solid with energy in their kinetic store
This breaks the rigid bonds between the particles meaning they can now flow over each other
What happens when a liquid is heated and evaporates
Thermal energy transfer takes place and supplies the particles on the surface of the liquid with energy in their kinetic store
This removes the bonds between the particles meaning they can move about randomly and spread far apart
Heating a systems will change the energy stored in a system by
Increasing the kinetic energy of its particles = change of state
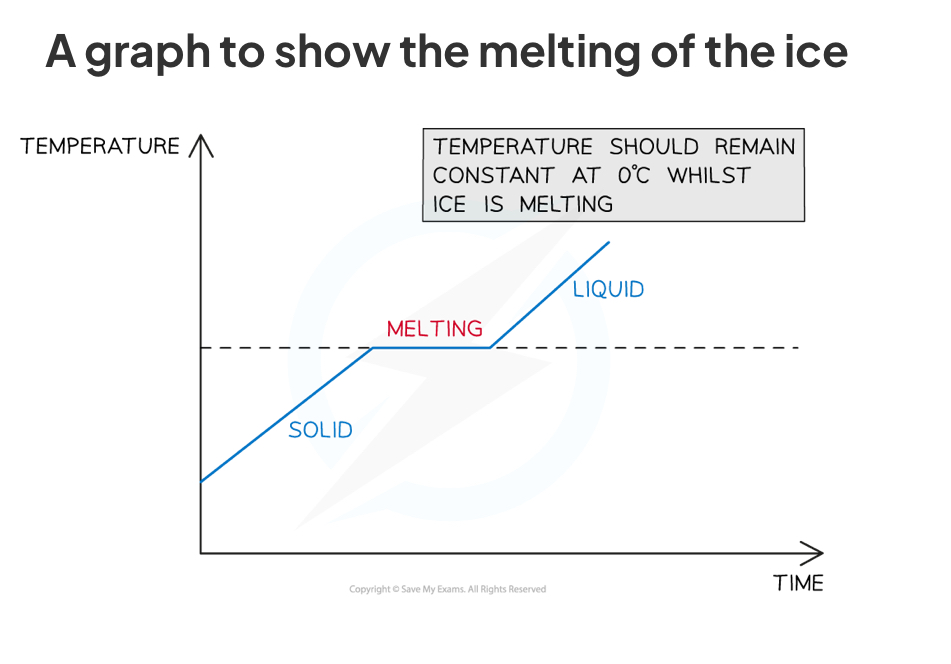
Practical : temp-time graph - changes of state
Place the ice cubes in the beaker (it should be about half full)
Place the thermometer in the beaker
Place the beaker on the tripod and gauze and slowly start to heat it using the bunsen burner
As the beaker is heated, take regular temperature measurements (e.g. at one minute intervals)
Continue this whilst the substance changes state (from solid to liquid)
Specific heat capacity
Is the energy required to change the temperature of an object by one degree Celsius per kilo of mass (J/kgC)
Change in thermal energy (J) =
Mass (kg) x specific heat capacity (J/kgC) x change in temp (C)
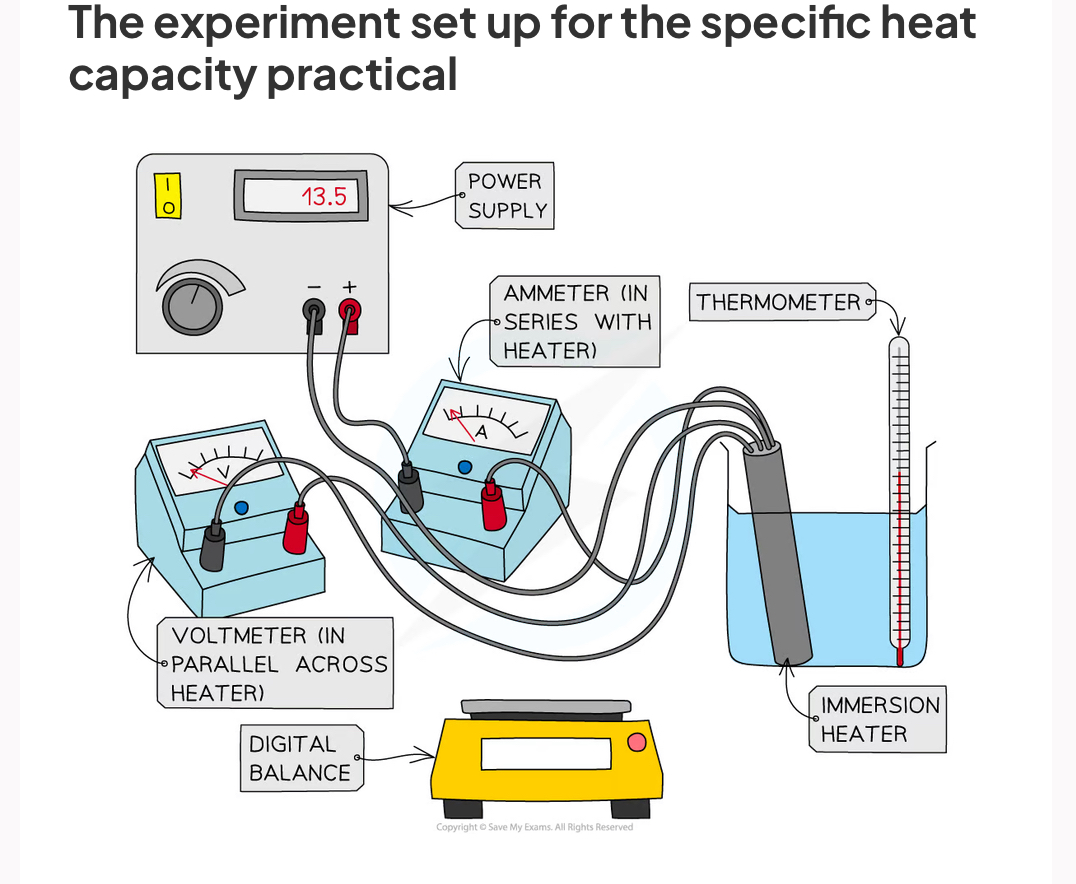
Practical : investigating specific heat capacity
Place the beaker on the digital balance and press 'zero'
Add approximately 250 ml of water and record the mass of the water using the digital balance
Place the immersion heater and thermometer in the water
Connect up the circuit as shown in the diagram, with the ammeter in series with the power supply and immersion heater, and the voltmeter in parallel with the immersion heater
Record the initial temperature of the water at time 0 s
Turn on the power supply, set it at approximately 10 V, and start the stopwatch
Record the voltage from the voltmeter and the current from the ammeter
Continue to record the temperature, voltage and current every 60 seconds for 10 minutes
Repeat steps 2-8, replacing the beaker of water for the solid block of aluminium and starting with recording its mass using the digital balance
Molecules in a gas have random motion and exert a force which means
They exert a force which causes a pressure on the walls of a container bcs as particles move randomly they collide with the walls of their containers = collisions produce net force against walls = gas at high pressure has more frequent collisions and a greater more frequent collisions so higher pressure = higher force exerted per unit area