chapter 11 ochem quiz
1/42
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
43 Terms
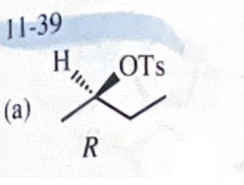
11-39 Predict the major products of the following reactions, including stereochemistry where appropriate.
(R)-butan-2-ol + TsCl in pyridine
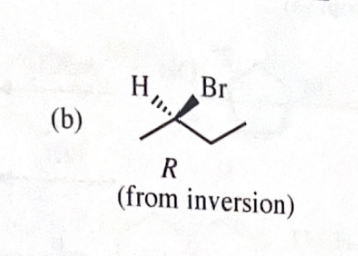
11-39 Predict the major products of the following reactions, including stereochemistry where appropriate.
(S)-2-butyl tosylate + NaBR
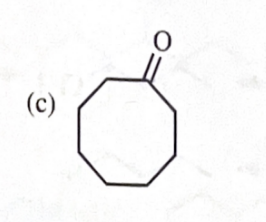
11-39 Predict the major products of the following reactions, including stereochemistry where appropriate.
cyclooctanol + Na OCl/HOAc
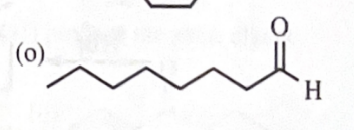
11-39 Predict the major products of the following reactions, including stereochemistry where appropriate.
octan-1-ol + DMSO + oxalyl chloride
11-39 Predict the major products of the following reactions, including stereochemistry where appropriate.
cyclopentylmethanol + CrO3 times pyridine times HCl
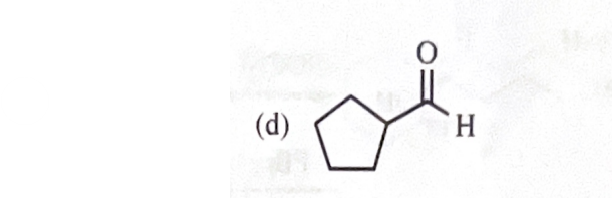
11-39 Predict the major products of the following reactions, including stereochemistry where appropriate.
cyclopentylmethanol + Na2Cr2O7/H2SO4
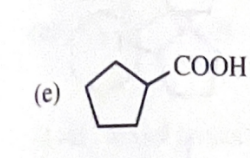
11-39 Predict the major products of the following reactions, including stereochemistry where appropriate.
cyclopentanol + HCl/ZnCl2
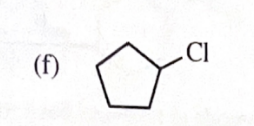
11-39 Predict the major products of the following reactions, including stereochemistry where appropriate.
n-butanol + HBr
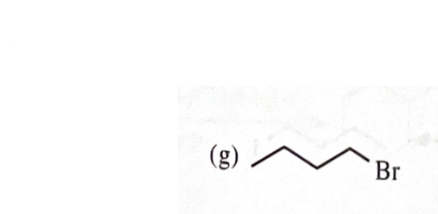
11-39 Predict the major products of the following reactions, including stereochemistry where appropriate.
cyclooctlymethanol + CH3CH2MgBr
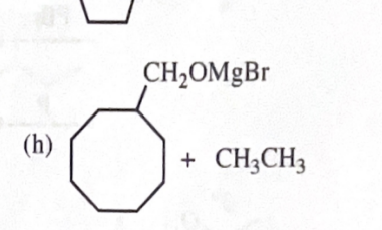
11-39 Predict the major products of the following reactions, including stereochemistry where appropriate.
potassium tert-butoxide + methyliodide
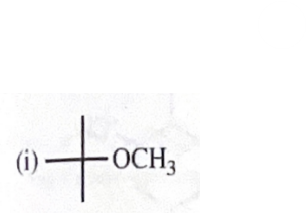
11-39 Predict the major products of the following reactions, including stereochemistry where appropriate.
sodium methoxide + tert - butyliodide
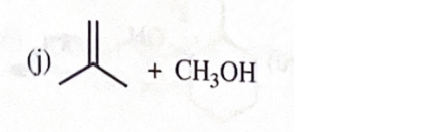
11-39 Predict the major products of the following reactions, including stereochemistry where appropriate.
cyclopentanol + H2SO4/heat

11-39 Predict the major products of the following reactions, including stereochemistry where appropriate.
product from (k) + OsO4H2O2, then HIO4
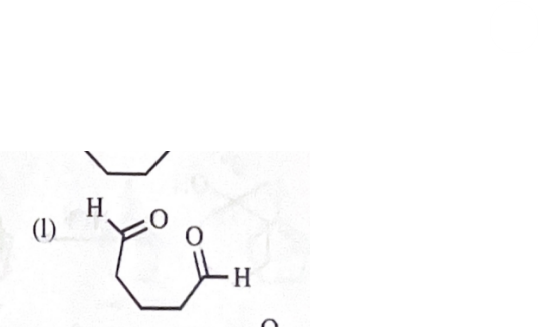
11-39 Predict the major products of the following reactions, including stereochemistry where appropriate.
sodium ethoxide +1 - bromobutane
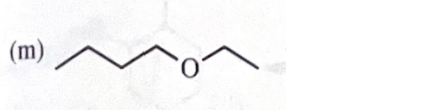
11-39 Predict the major products of the following reactions, including stereochemistry where appropriate.
sodium ethoxide + 2 - methyl - 2 - bromobutane
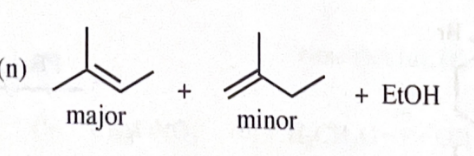
11-39 Predict the major products of the following reactions, including stereochemistry where appropriate.
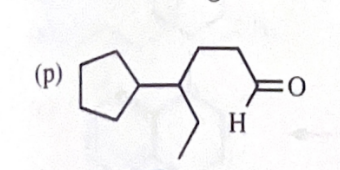
4-cylcopentylhexan-1-ol + DMP reagent
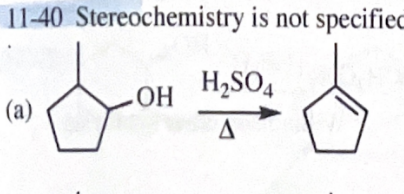
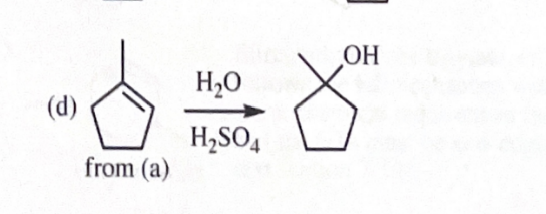
11-40 Show how you would convert 2-methylcyclopentanol to the following products. Any of these products may be used as the reactant in any subsequent part of this problem.
1-methylcyclopentene
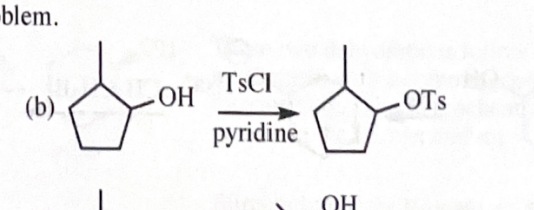
11-40 Show how you would convert 2-methylcyclopentanol to the following products. Any of these products may be used as the reactant in any subsequent part of this problem.
2-methylcyclopentyl tosylate
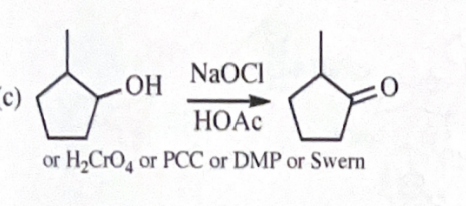
11-40 Show how you would convert 2-methylcyclopentanol to the following products. Any of these products may be used as the reactant in any subsequent part of this problem.
2-methylcyclopentanone
11-40 Show how you would convert 2-methylcyclopentanol to the following products. Any of these products may be used as the reactant in any subsequent part of this problem.
1-methylcyclopentanol
11-40 Show how you would convert 2-methylcyclopentanol to the following products. Any of these products may be used as the reactant in any subsequent part of this problem.
1,2-dimethylcyclopentanol
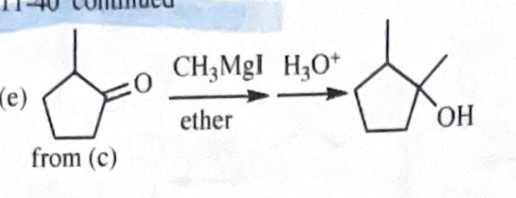
11-40 Show how you would convert 2-methylcyclopentanol to the following products. Any of these products may be used as the reactant in any subsequent part of this problem.
1-bromo-2-methylcyclopentane
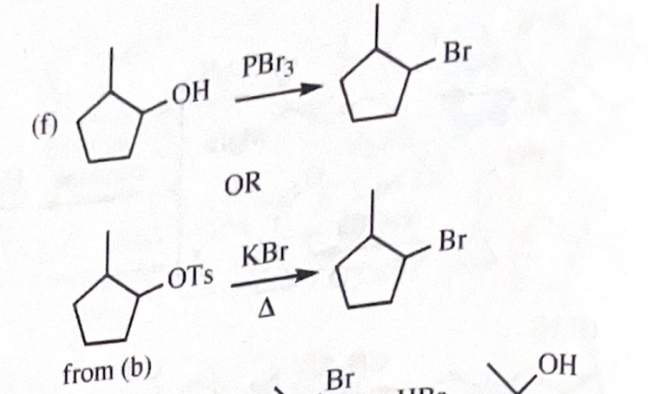
11-40 Show how you would convert 2-methylcyclopentanol to the following products. Any of these products may be used as the reactant in any subsequent part of this problem.
2-methylcyclopentyl acetate
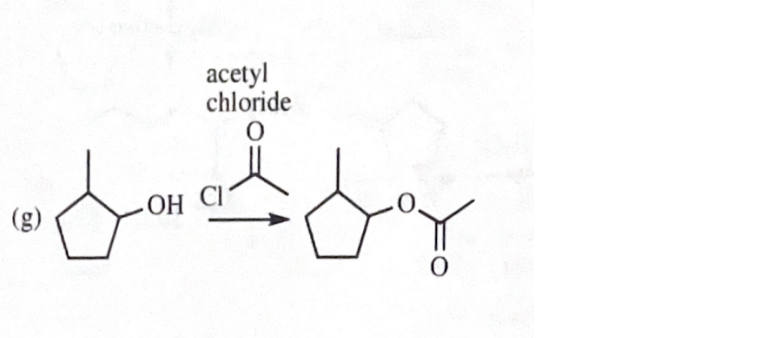
11-40 Show how you would convert 2-methylcyclopentanol to the following products. Any of these products may be used as the reactant in any subsequent part of this problem.
1-bromo-1-methylcyclopentane
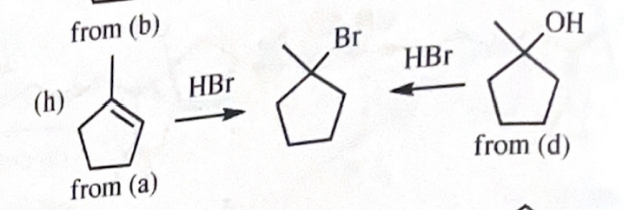
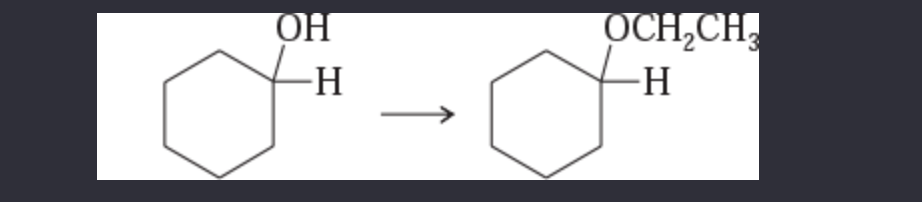
11-42 Show how you would accomplish the following synthetic conversions.
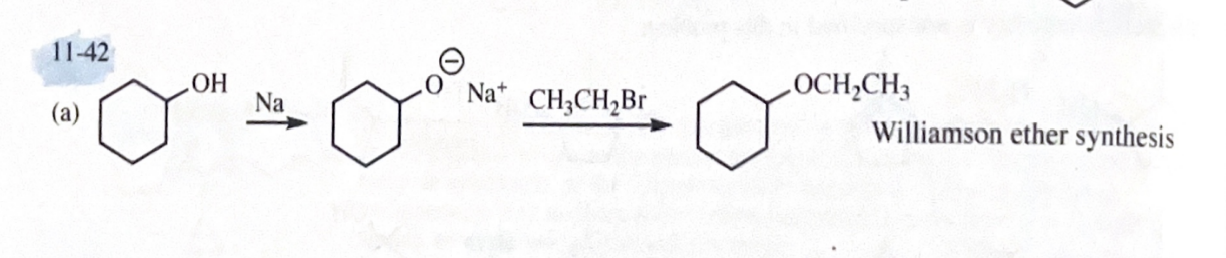
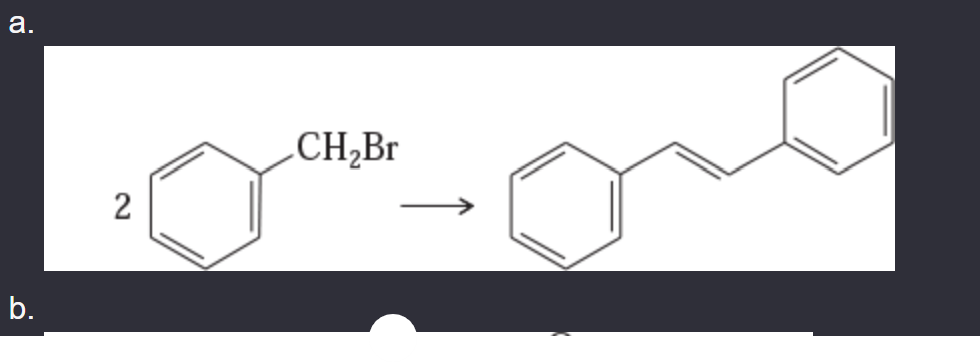
11-42 Show how you would accomplish the following synthetic conversions.
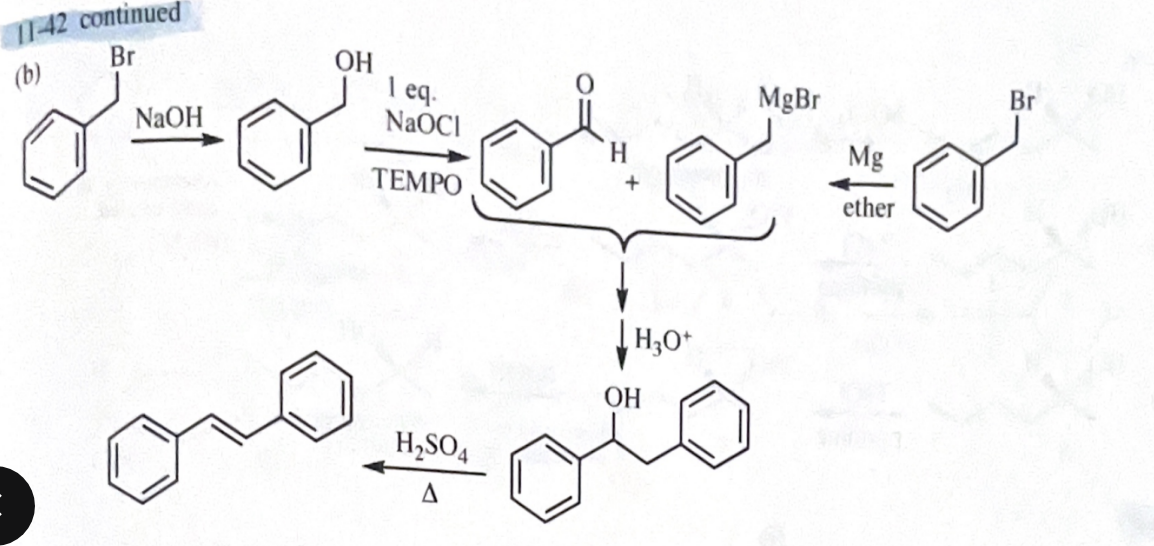
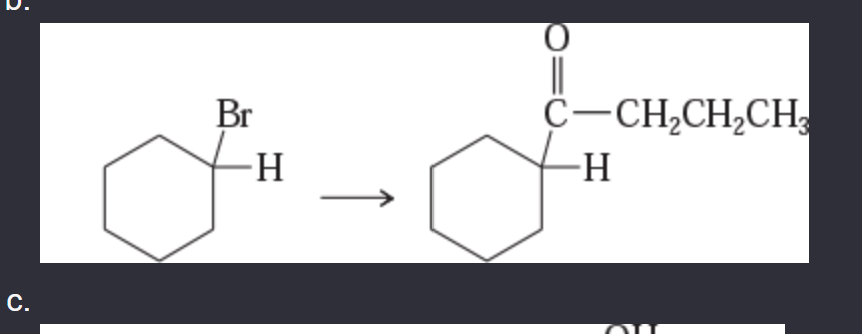
11-42 Show how you would accomplish the following synthetic conversions.
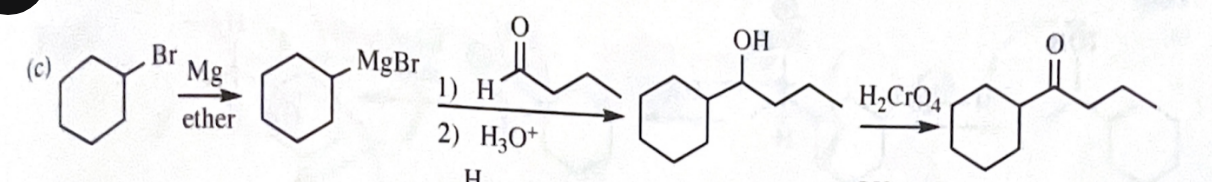
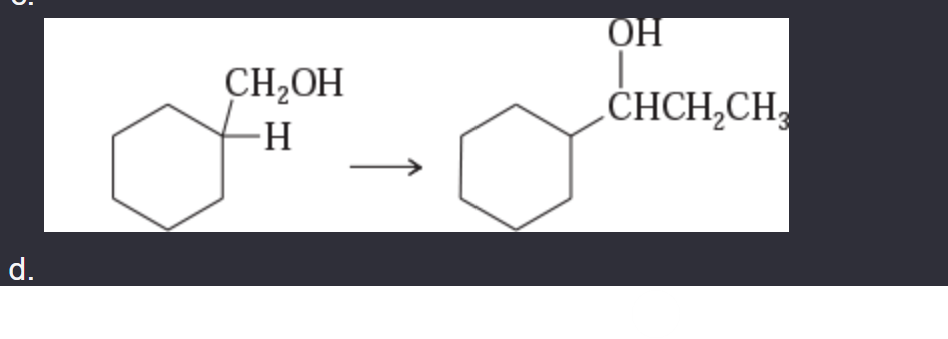
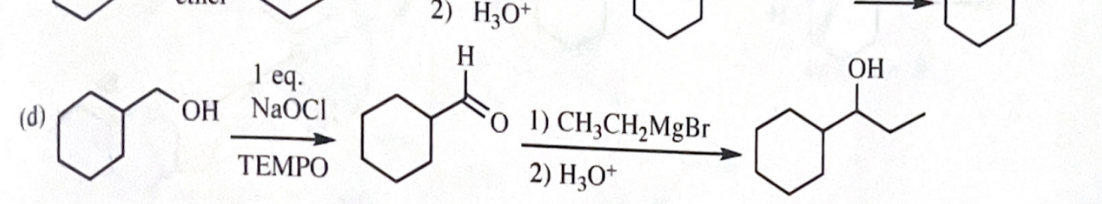
11-42 Show how you would accomplish the following synthetic conversions.
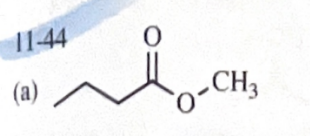
11-44 Predict the esterification products of the following acid/alcohol pairs.
CH3CH2CH2COOH + CH3OH
11-44 Predict the esterification products of the following acid/alcohol pairs.
CH3OH + HNO3
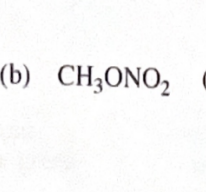
11-44 Predict the esterification products of the following acid/alcohol pairs.
2 CH3CH2OH + H3PO4
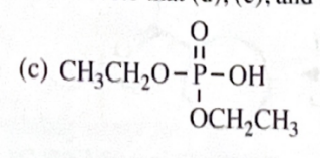
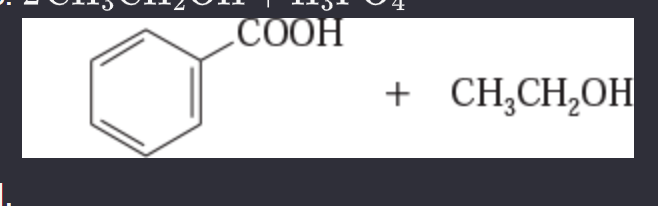
11-44 Predict the esterification products of the following acid/alcohol pairs.
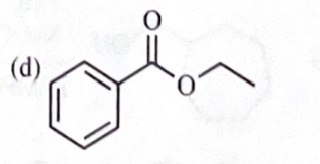
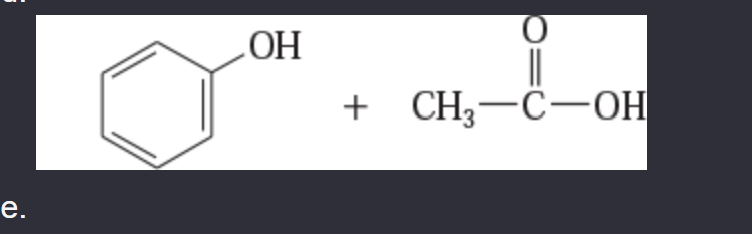
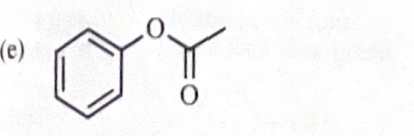
11-44 Predict the esterification products of the following acid/alcohol pairs.
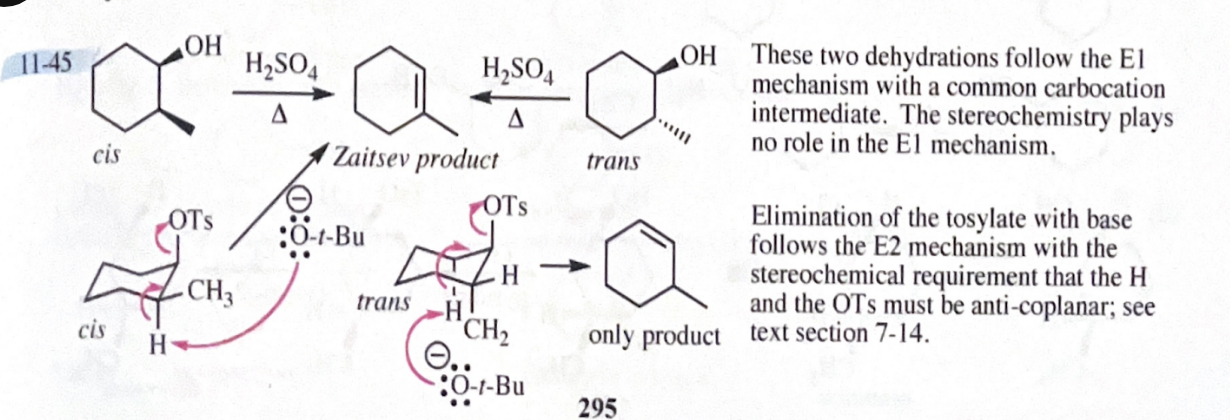
11-45 Both cis- and trans-2-methylcyclohexanol undergo dehydration in warm sulfuric acid to give 1-methylcyclohexene as the major alkene product. These alcohols can also be converted to alkenes by tosylation using TsCl and pyridine, followed by elimination using KOC(CH3)3 as a strong base. Under these basic conditions, the tosylate of cis-2-methylcyclohexanol eliminates to give mostly 1-methylcyclohexene, but the tosylate of trans-2-methylcyclohexanol eliminates to give only 3-methylcyclohexene. Explain how this stereochemical difference in reactants controls a regiochemical difference in the products of the basic elimination, but not in the acid-catalyzed elimination.
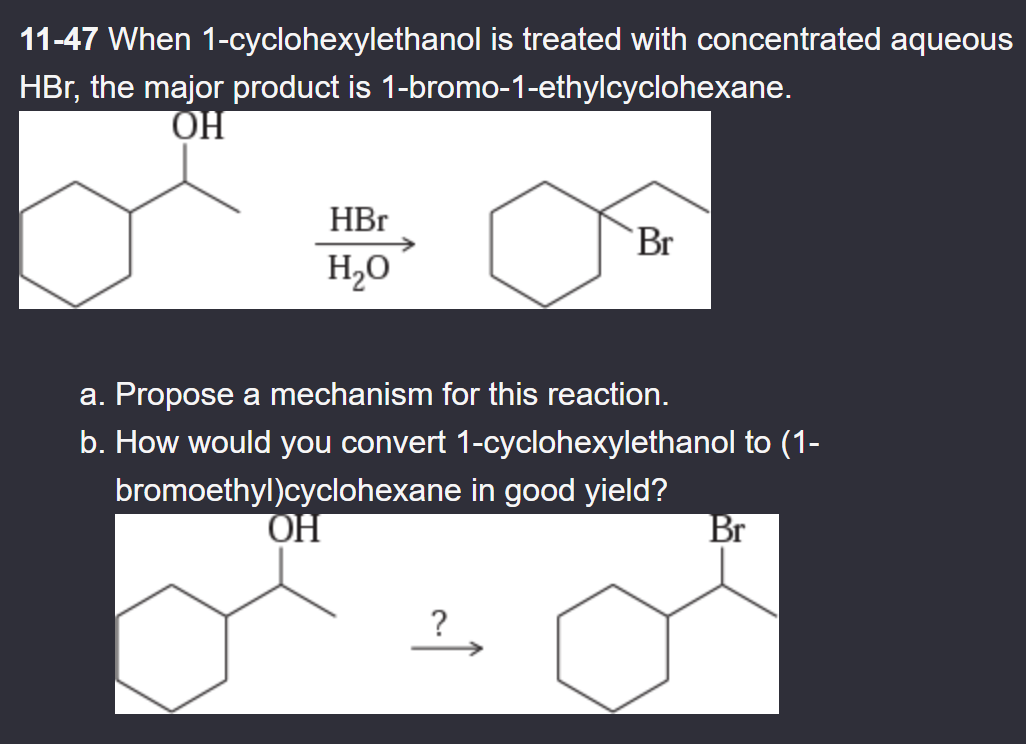
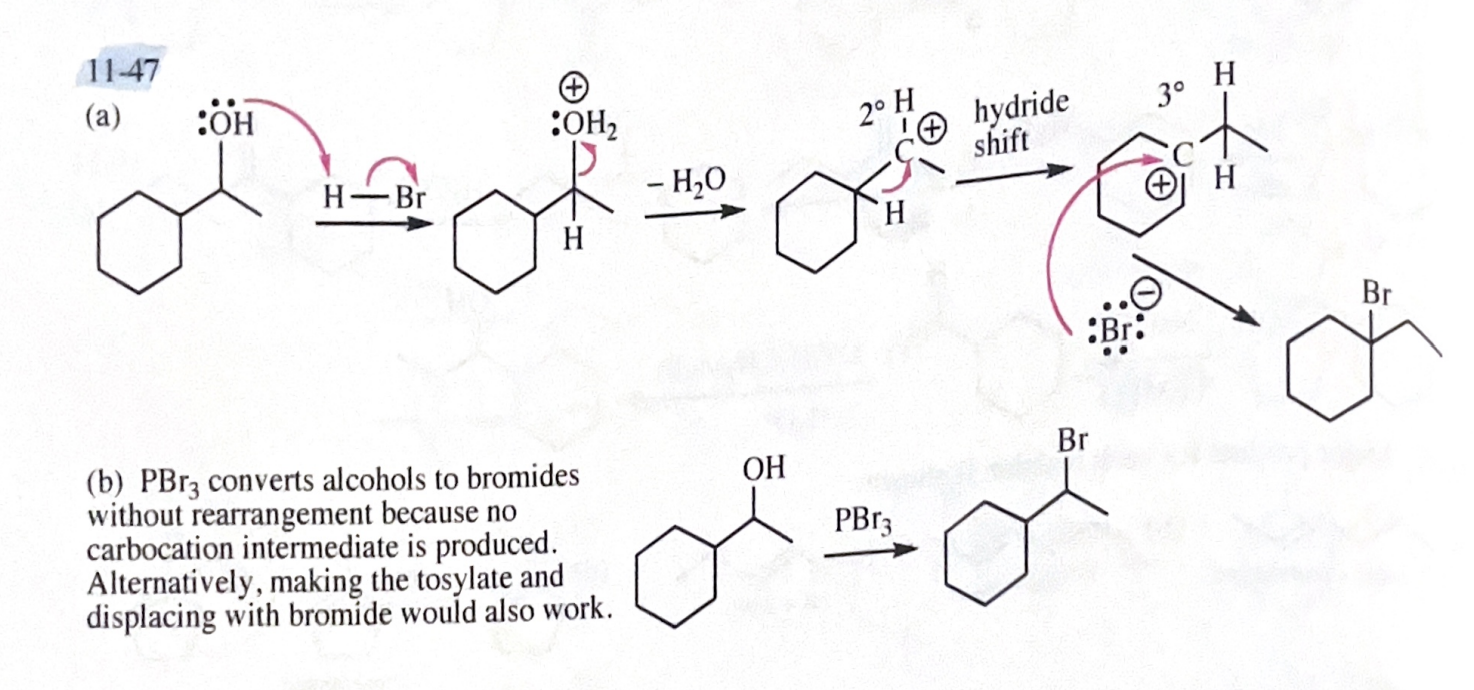
11-49 Predict the major products (including stereochemistry) when cis-3-methylcyclohexanol reacts with the following reagents.
PBr3
SOCl2
Lucas reagent
concentrated HBr
TsCl/pyridine, then NaBr
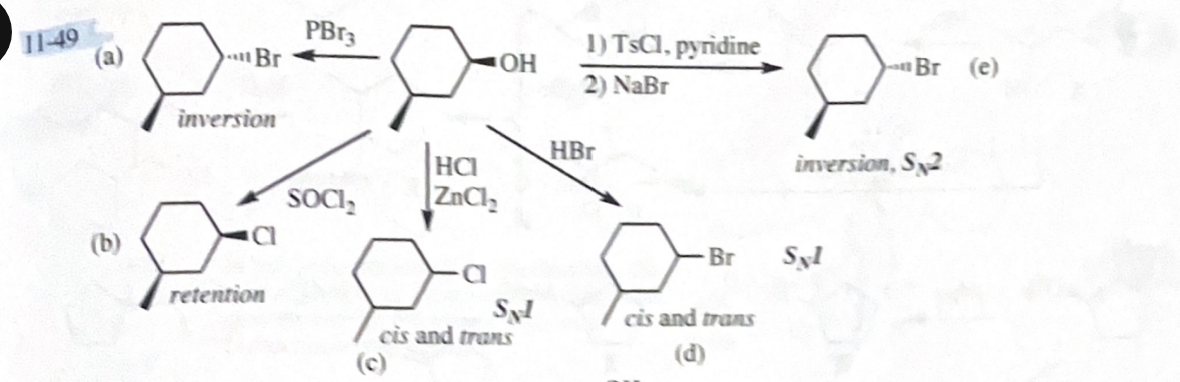
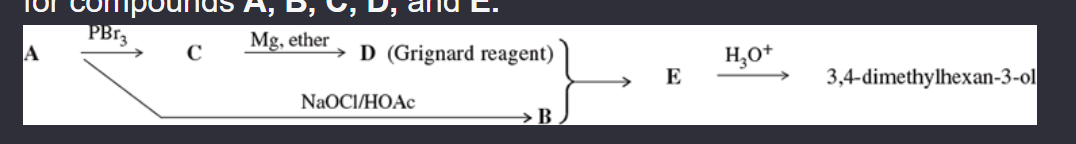
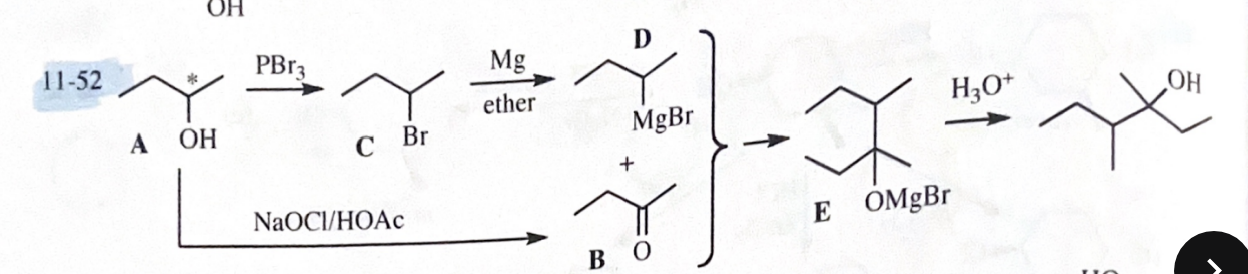
11-52 Compound A is an optically active alcohol. Treatment with chromic acid converts A into a ketone, B. In a separate reaction, A is treated with PBr3 converting A into compound C. Compound C is purified, and then it is allowed to react with magnesium in ether to give a Grignard reagent, D. Compound B is added to the resulting solution of the Grignard reagent. After hydrolysis of the initial product (E), this solution is found to contain 3,4-dimethylhexan-3-ol. Propose structures for compounds A, B, C, D, and E.
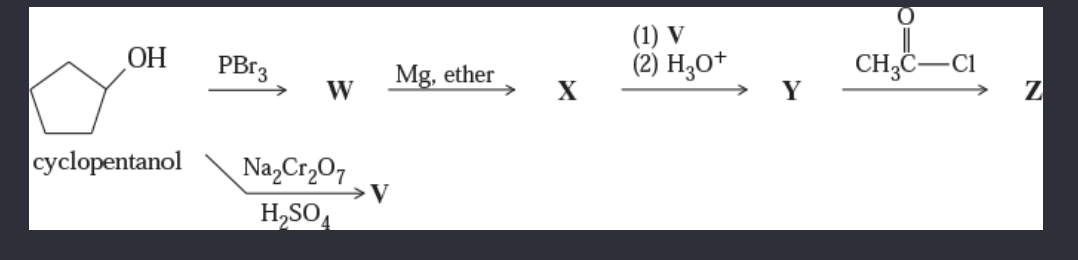
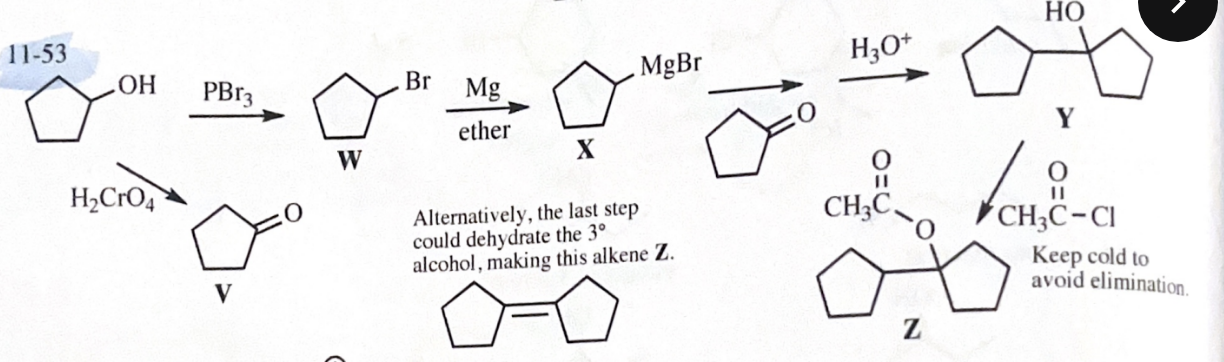
11-53 Give the structures of the intermediates and products V through Z.
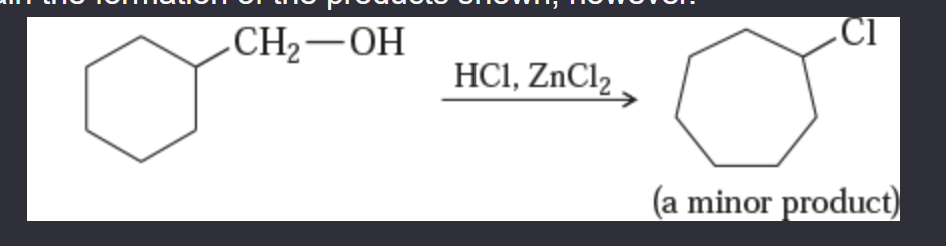
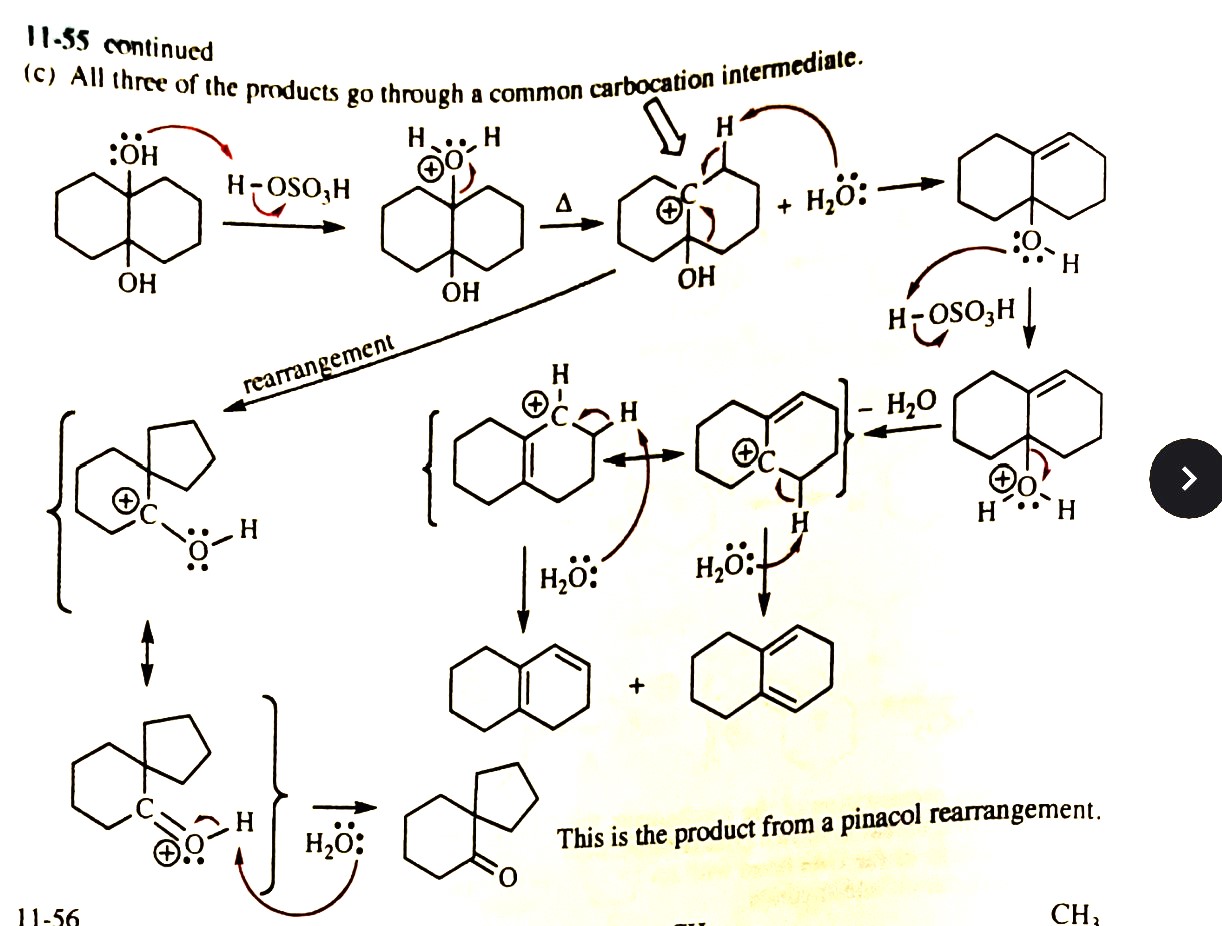
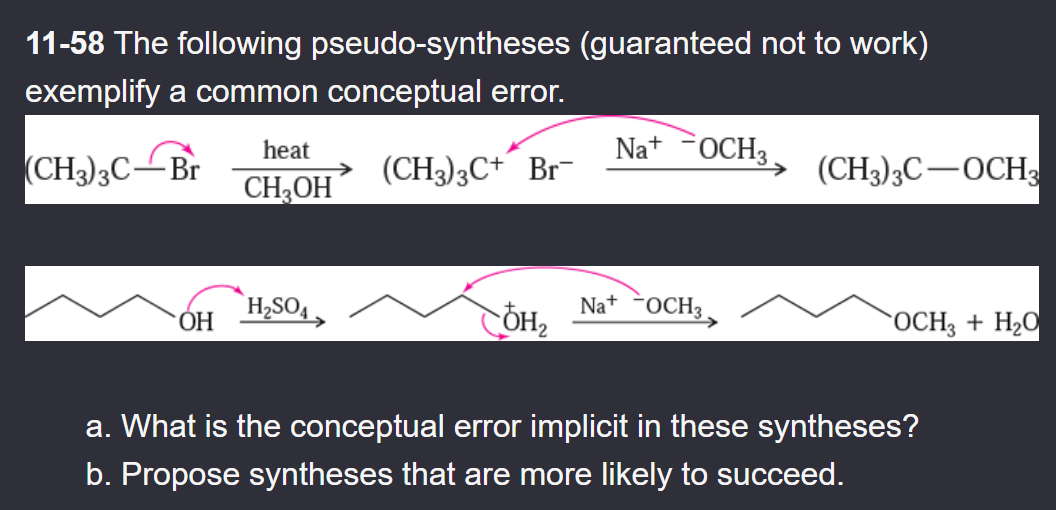
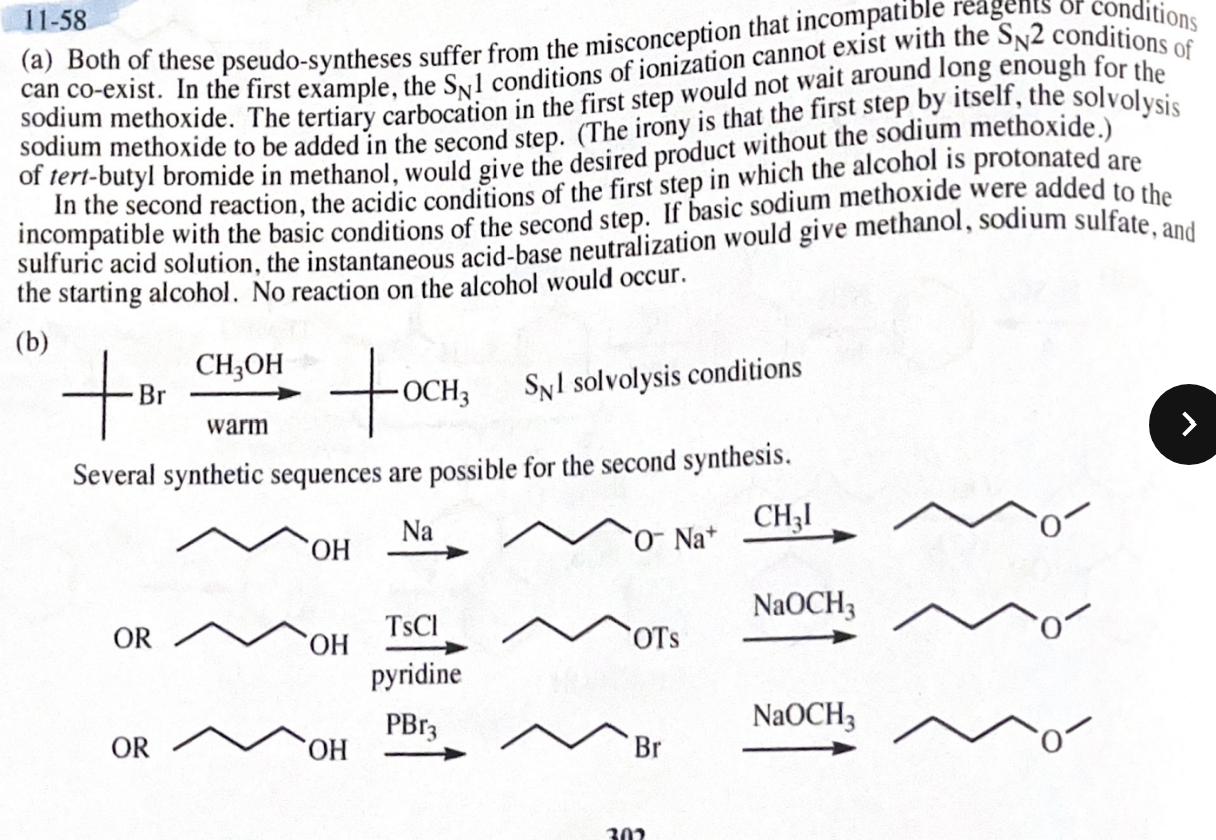
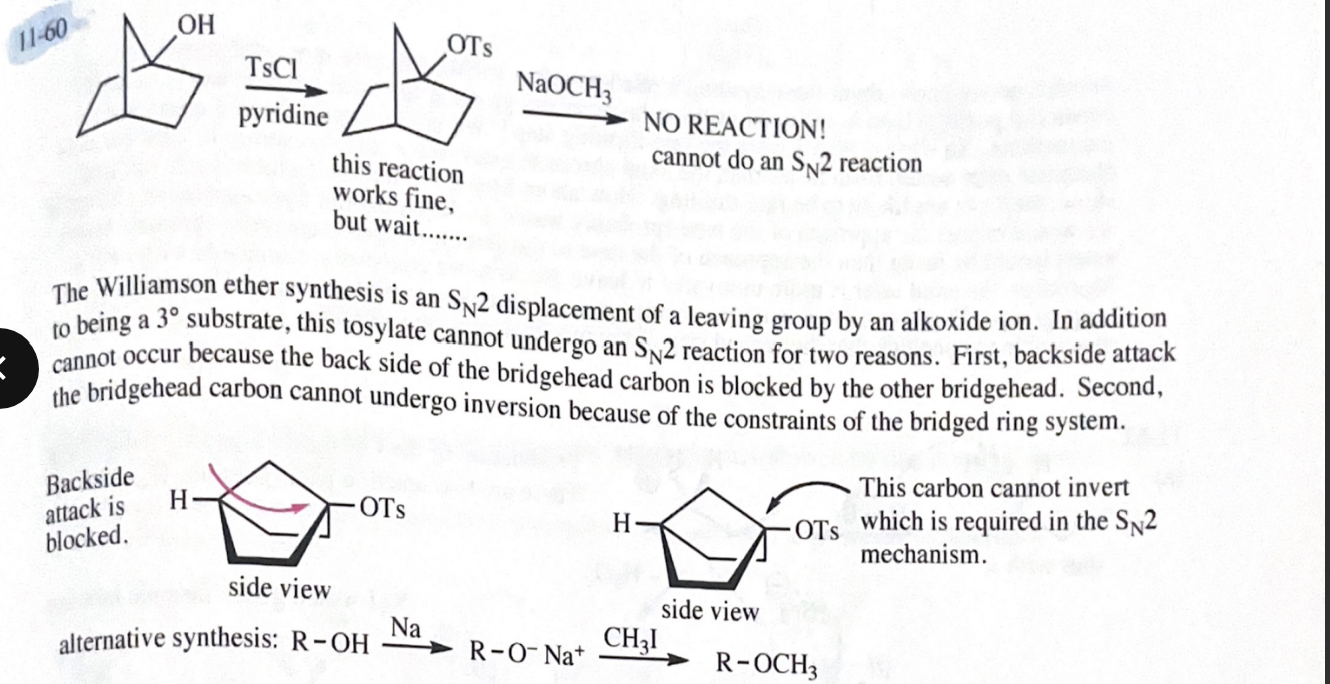
11-55 Propose mechanisms for the following reactions. In most cases, more products are formed than are shown here. You only need to explain the formation of the products shown, however.
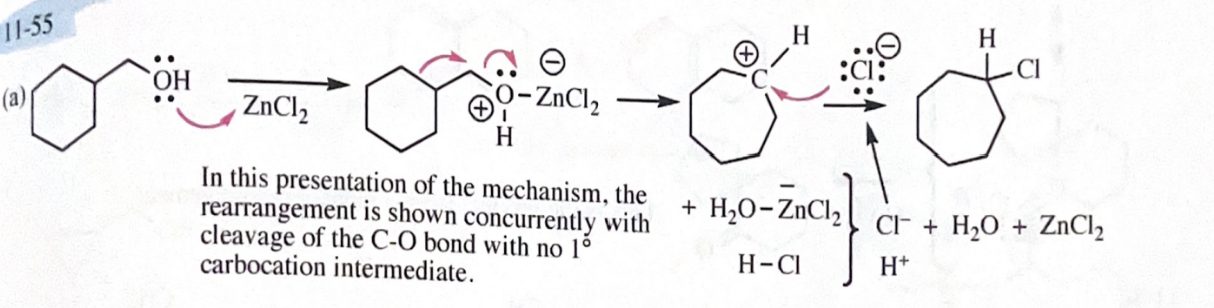
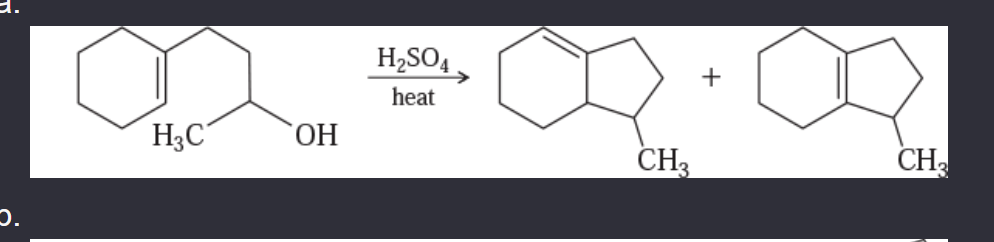
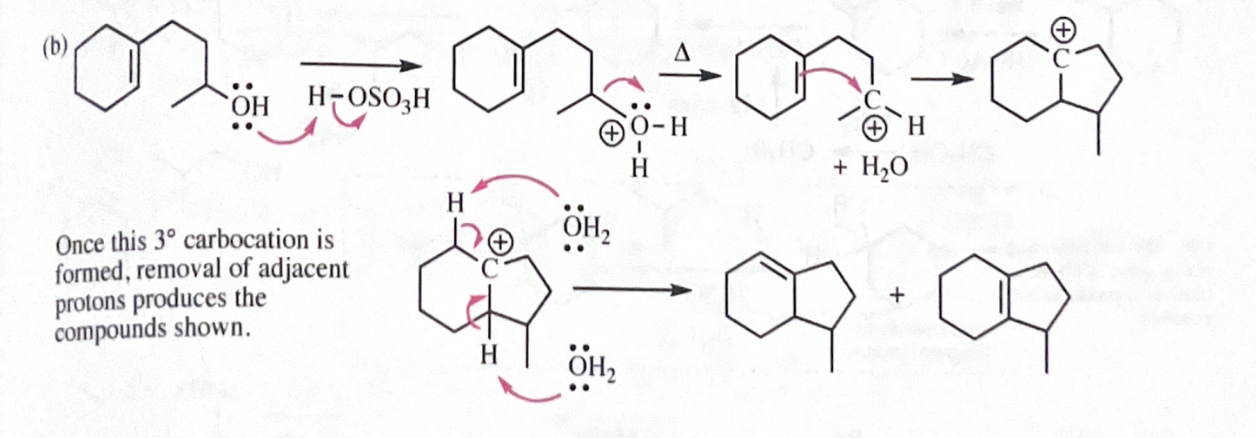
11-55 Propose mechanisms for the following reactions. In most cases, more products are formed than are shown here. You only need to explain the formation of the products shown, however.
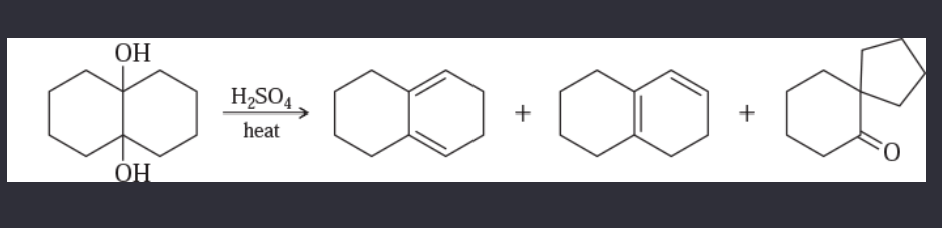
11-55 Propose mechanisms for the following reactions. In most cases, more products are formed than are shown here. You only need to explain the formation of the products shown, however.