Structure 1.1.2: Kinetic molecular theory
1/7
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
8 Terms
plasma
An ionised gas mainly found in outer space.
Density
the ratio of its mass to its volume
kinetic molecular theory (KMT)
All matter is made up of small particles.
These particles all have kinetic energy (the energy of motion) which causes the particles to constantly move.
The amount of kinetic energy is proportional to the temperature of the substance; therefore, the particles have greater motion at higher temperatures (straight line motion) and lesser motion at lower temperatures (vibrational motion).
Collisions between particles are elastic, which means no loss in kinetic energy.
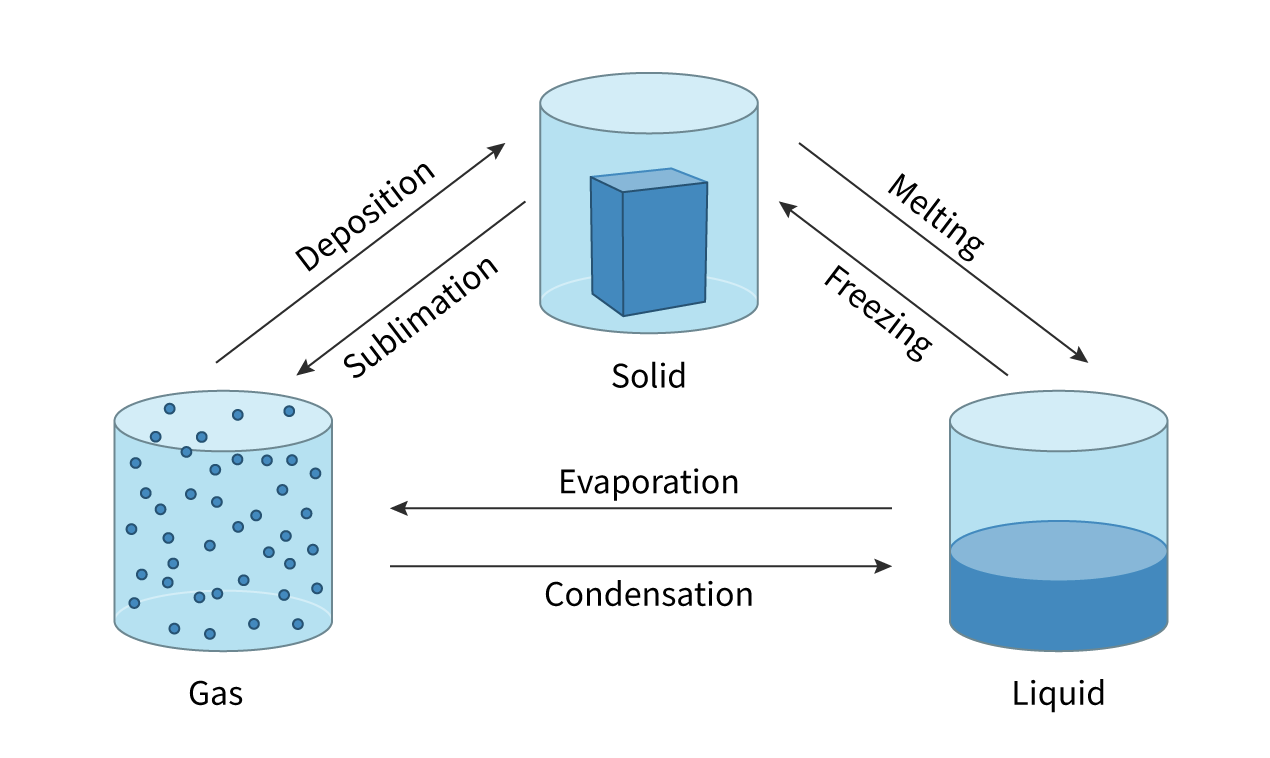
Sublimation
change of state directly from a solid to a gas with no liquid phase
Deposition
the direct change from a gas to solid with no liquid phase
Evaporation
change of state from liquid to gas and takes place only at the surface of the liquid. It can occur at temperatures below the boiling point of the liquid
Boiling
change of state from liquid to gas throughout the liquid. When the vapour pressure is equal to the
external pressure (1 atm) the liquid boils