6: biophysical and diffraction
1/120
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
121 Terms
describe spectroscopic vs diffraction methods
spectroscopy
rely on transition between energy levels
vary wavelength of radiation = absorption/emission occurs at given frequencies
sample (typically) in solution
diffraction
interaction with matter
keep wavelength fixed = measure variations in intensities with incident direction
sample in solid state
simply describe x-ray diffraction
x-rays = similar wavelength to the spacing between atoms in sample
x-rays are focused on sample. x-ray are reflected by electrons surrounding atoms of the sample, changing the direction by theta. these scattered x-rays constructively interfere to produce a diffraction pattern.
describe diffraction on different types of material
i.e. fibres, single crystals, powders
product different diffraction patterns = same theory applies to interpret them
what range are x-rays?
0.01 → 10 nm
~ distances between atoms
what kind of scattering is x-ray diffraction?
elastic
energy maintained
direction altered
describe the photoelectric effect
when a photon strikes an atom/molecule and transfers its energy to inner-shell electron.
if the photon has sufficient energy (≥ electron binding energy), the electron is ejected from its shell
ejected electron = photoelectron
describe the generation of x-rays
x-ray tube containing:
cathode (-ve) = source of electrons ~ tungsten
metal anode (+ve) = target ~ copper
vacuum
a current is passed through the cathode, which heats up and emits electrons. these electrons are accelerated through the vacuum to target, gaining kinetic energy. when electron hits the anode target, kinetic energy is converted to:
heat (major): anode must be cooled due to release of heat
x-rays (minor): if the incident electron has enough energy, it can knock out 1s electron from the K-shell (innermost electron shell) of the anode atom = ionisation. K-shell vacancy is filled from a higher energy shell (L-shell/M-shell), releasing energy in the form of an x-ray (photons with x-ray wavelength)
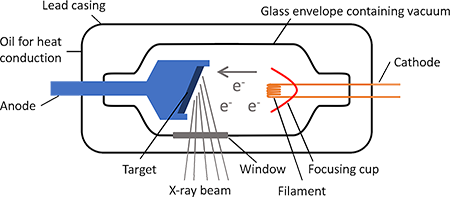
what are the two types of x-rays which can be produced by x-ray tubes?
characteristic x-rays
Bremsstrahlung radiation
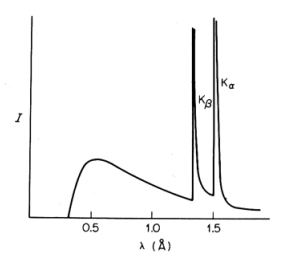
what are characteristic x-rays?
x-rays produced by x-ray tube
= energy/wavelength is specific to size of X→K transition
= energy/wavelength is specific to anode element
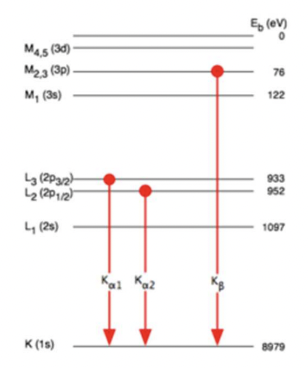
what are the different shell labels?
K: n=1
L: n=2
M: n=3
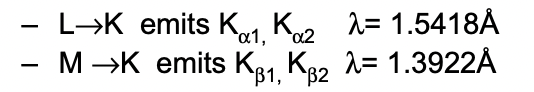
what gives rise to the different types of K radiation?
due to SO coupling splitting the degenerate 2p/3p orbitals
= different size transitions
describe Bremsstrahlung radiation
when electron decelerates rapidly as they interact with electric field of the nucleus, they can produce broad-spectrum X-rays that can vary in wavelength and are not specific to the anode element
what is the general equation for x-ray absorption? *not given*
I = intensity of radiation after passing through material
I(0) = intensity of incident radiation
µ = absorption coefficient of the material (dependent on material and X-ray wavelength)
t = path length

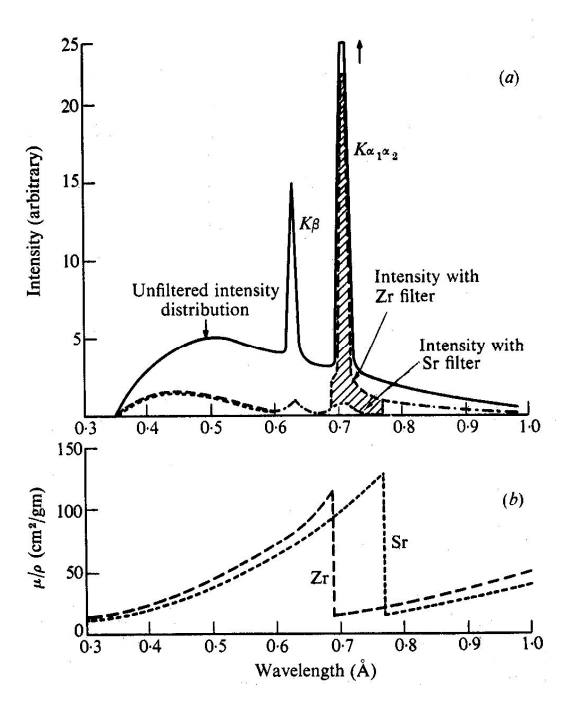
describe filters
selectively remove unwanted x-ray radiation from spectrum, particular K(B)
K(B) = contributes to background noise/reduce image clarity
= have large absorption coefficients, µ, at certain wavelengths corresponding to K(B)
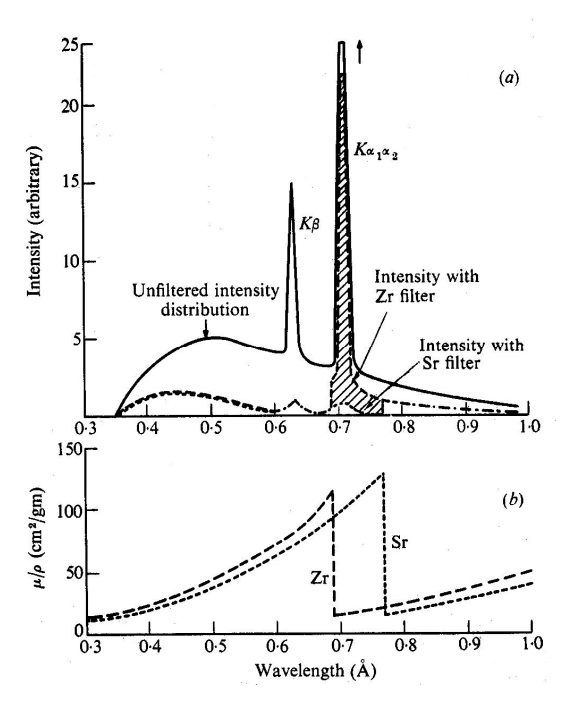
describe absorption edges
sudden increase in absorption of X-rays (absorption coefficient) above threshold energy to excite an electron from an inner atomic shell
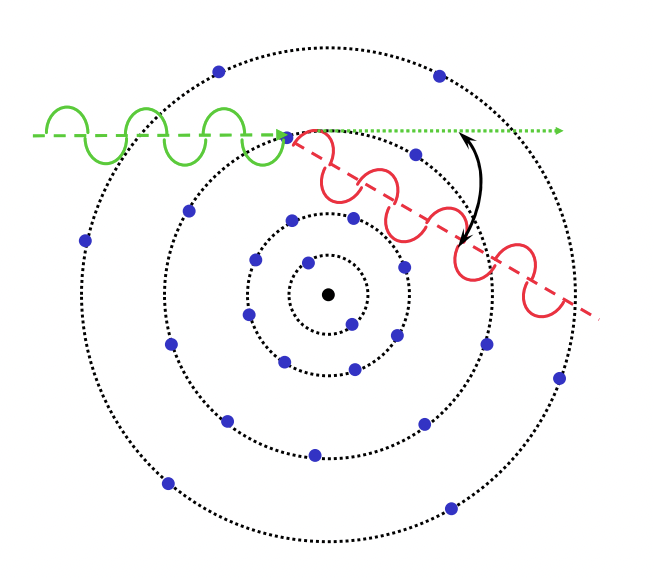
what are 3 types of x-ray interaction with material
Thompson scattering
Compton scattering
photoelectron absorption
describe scattering
the redirection of incoming radiation (photon in x-ray) due to interaction with matter (electrons in x-ray)
describe Thompson scattering
wave:
elastic scattering = no loss of energy
coherent scattering = no change in frequency/wavelength of photon = no energy transfer between photon and electron
wave is scattered in all directions = varying angles
electron:
no electron is ejected
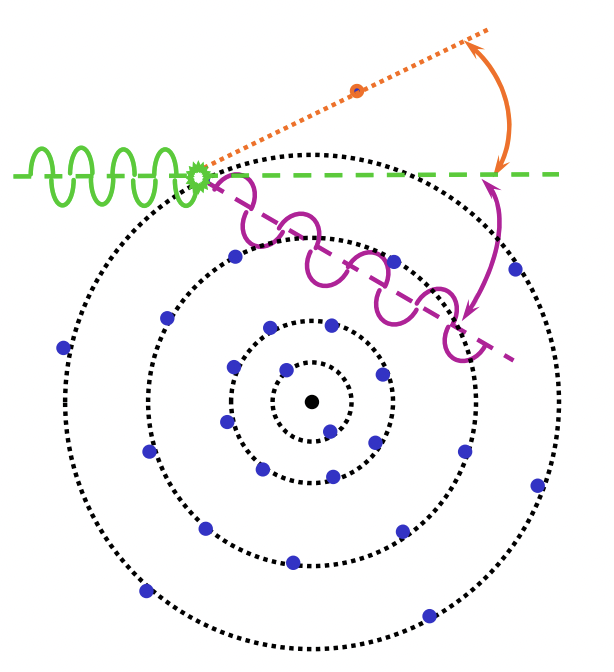
describe Compton scattering
wave:
inelastic scattering = loss of energy
incoherent scattering = decrease of frequency/increase in wavelength = energy transfer between photon and electron
wave is scattered in all direction = varying angles
electron:
outer shell (loosely bound) electron is ejected
describe photoelectron absorption
wave:
inelastic scattering = loss of energy
no scattering = complete energy transfer between photon and electron
no wave
electron:
inner shell electron is ejected = photoelectron
excess absorbed energy released as:
x-ray fluorescence (not scattered) = photoelectric effect
Auger electron = energy transferred to another electron and ejected
why are crystals used in x-ray diffraction?
electron scatter x-rays very weakly
= use ordered arrays of molecules
define structural units
structural unit = repeated by translation in 3 dimensions = all orientated identically
crystal lattice structure can be represented by structural units as single points
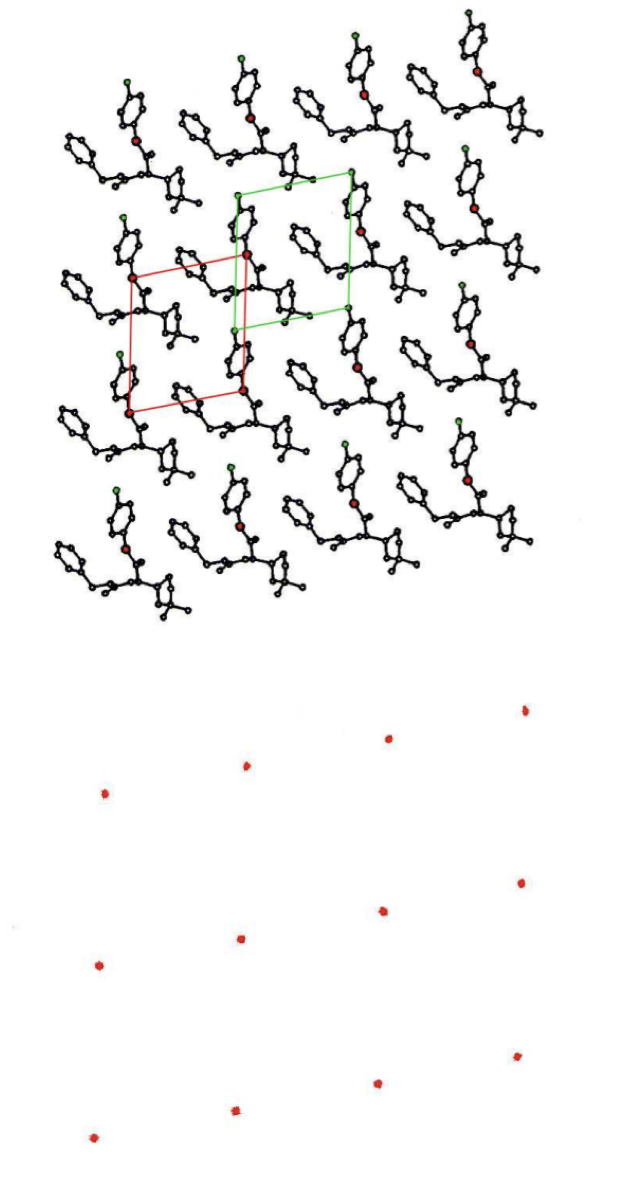
describe unit cell
smallest repeating unit of the lattice
parallelepiped (6 parallelogram-faced structure) with:
three edges (a, b, and c i.e. x, y, and z)
three angles (a, B, and Y)
convention:
a < b
Y ~ 90°
contains 1 whole molecule in total
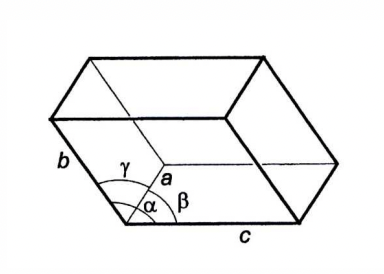
what are the 14 distinct lattice types which symmetry operations yield?
Bravias lattices
what are the 7 crystal systems of Bravias lattices?
triclinic
monoclinic
orthorhombic
tetragonal
rhombohedral
hexagonal
cubic
what are the different labels within each crystal system?
P = primitive = only points in each corner
I = body-centred
F = centred in all 6 faces
C = centred on 2 opposite faces
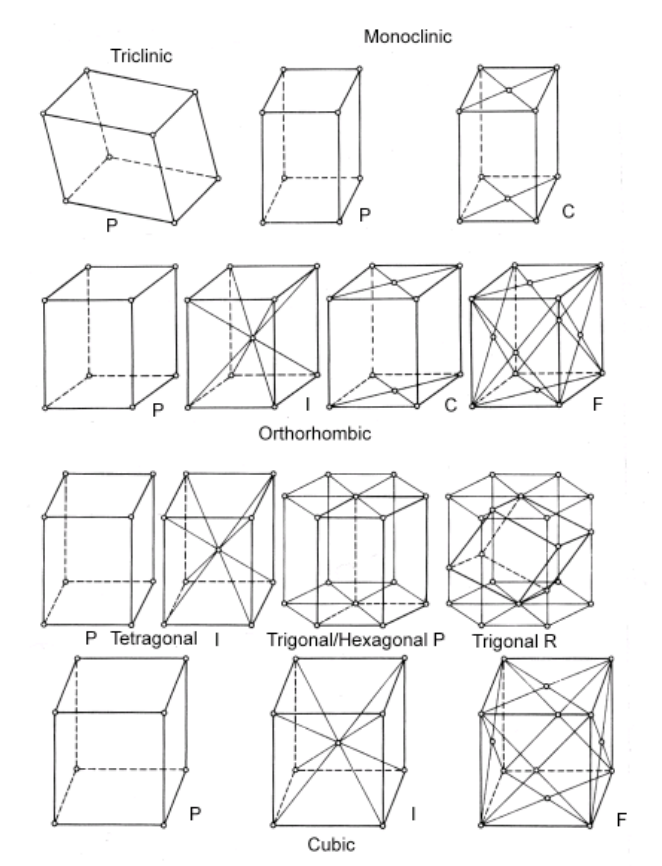
define asymmetric unit
smallest section of the unit cell/crystal structure related by symmetry
summarise crystalline structure
asymmetric unit -(space group)→ unit cell → crystal
space group = symmetry operations which generate unit cell from asymmetric unit
what are 3 types of interference?
intermediate
constructive
destructive
describe constructive interference
waves in phase: phase difference = 0
total reinforcement
A = single wave + single wave = 2*single wave
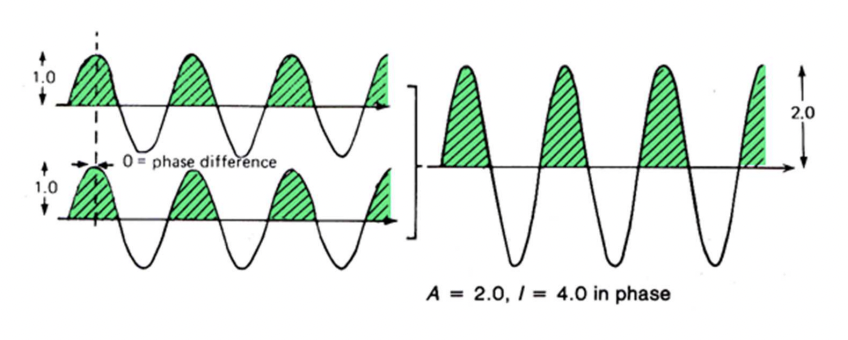
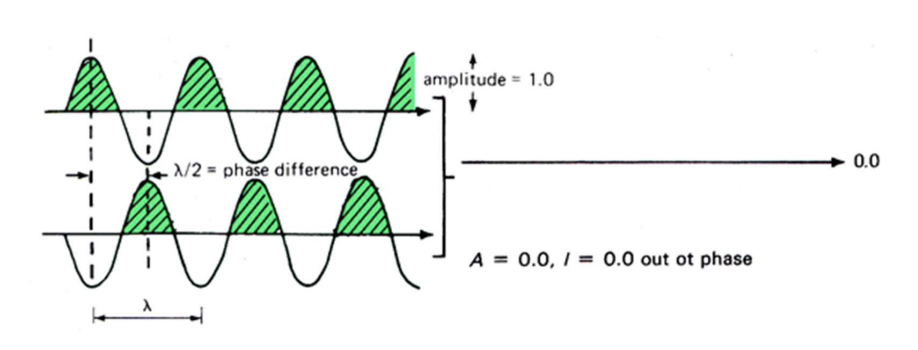
describe destructive interference
waves out of phase: phase difference = λ/2 or 180°
complete cancellation
no resultant wave
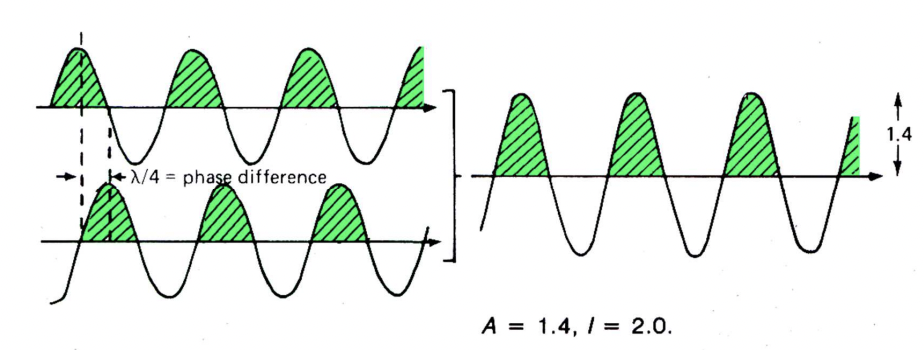
describe intermediate interference
waves partially in phase: i.e. phase difference = λ/4 or 90°
partial reinforcement
what kind of interference is required for x-ray diffraction?
constructive interference
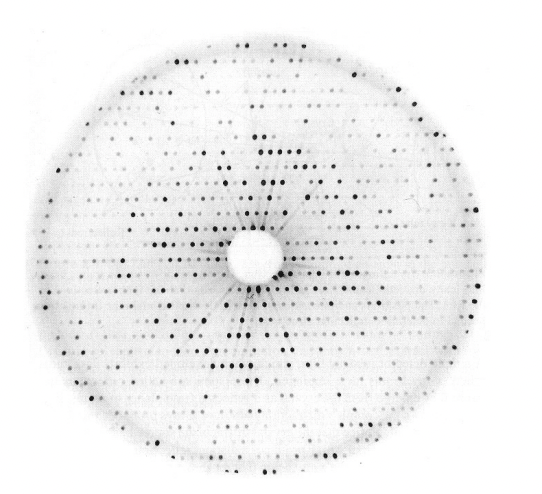
describe the features of a diffraction pattern
geometry
intensities
symmetry (of intensities)
describe Braggs law
incident x-ray onto a crystal surface at angle θ will be reflected by different crystal ‘planes’ by angle θ.
when constructive interference of scattered x-rays occurs, the scattered x-rays are intense enough to be visible on the diffraction pattern.
when constructive interference does not occur, the scattered x-rays partially or completely destructively interfere.
Braggs law defines when constructive interference occurs
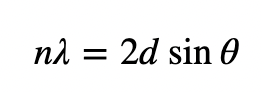
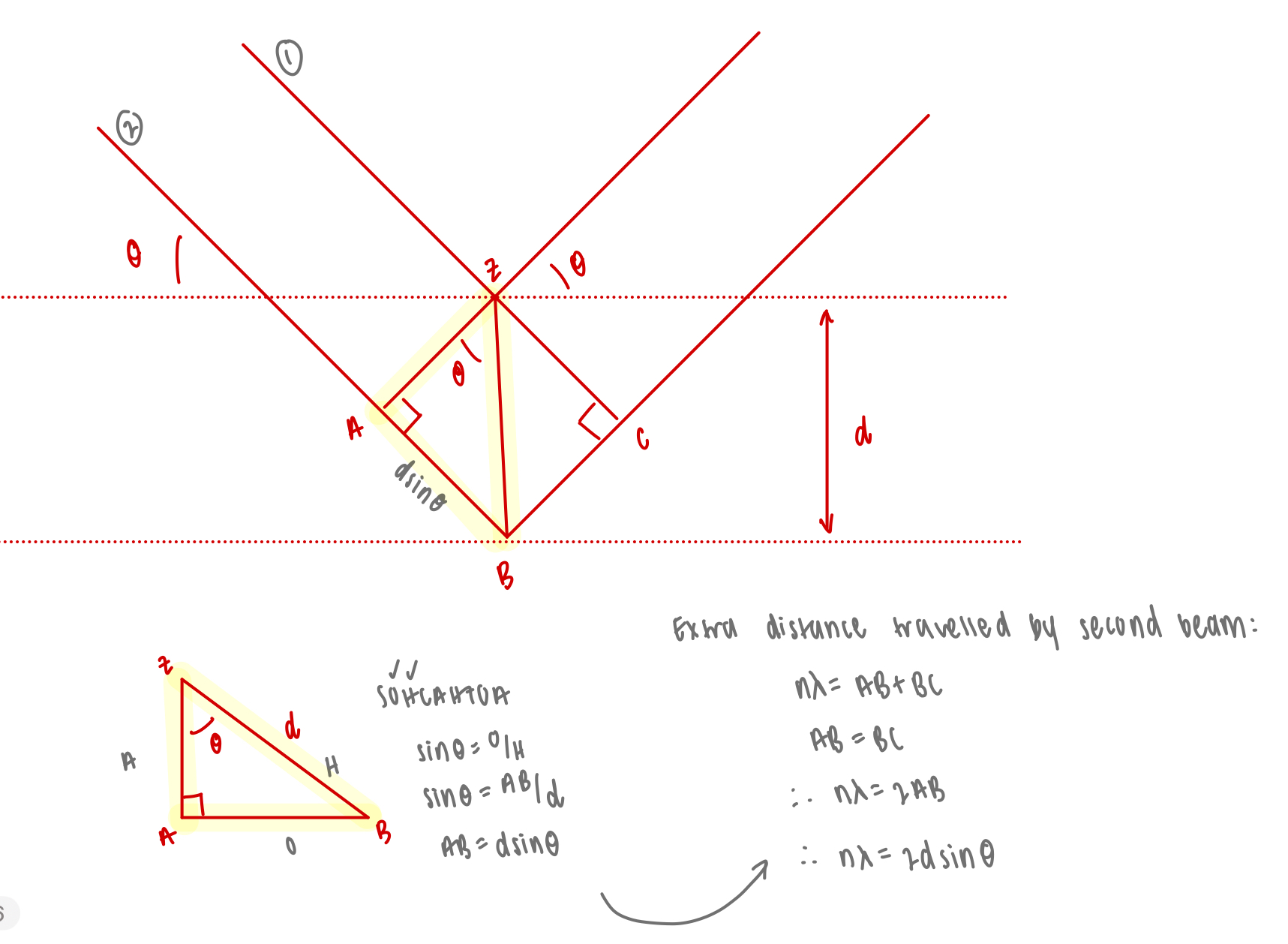
explain the derivation of Braggs law
constructive interferences will arise where the path difference is integer values of wavelength = Braggs law
describe crystal planes
simple molecules = convenient
complex molecules = difficult to see how they arise
describe scattering from point
lattice points are used as reference rather than planes
d = distance between lattice points
describe first order vs higher order diffraction
first order: n=1: path difference = λ
higher order: n>1: path difference = 2λ, 3λ, etc.
how does the angle of diffraction vary for different orders of diffraction
higher order of diffraction = larger n = larger angle of diffraction
lower order of diffraction = smaller n = smaller angle of diffraction
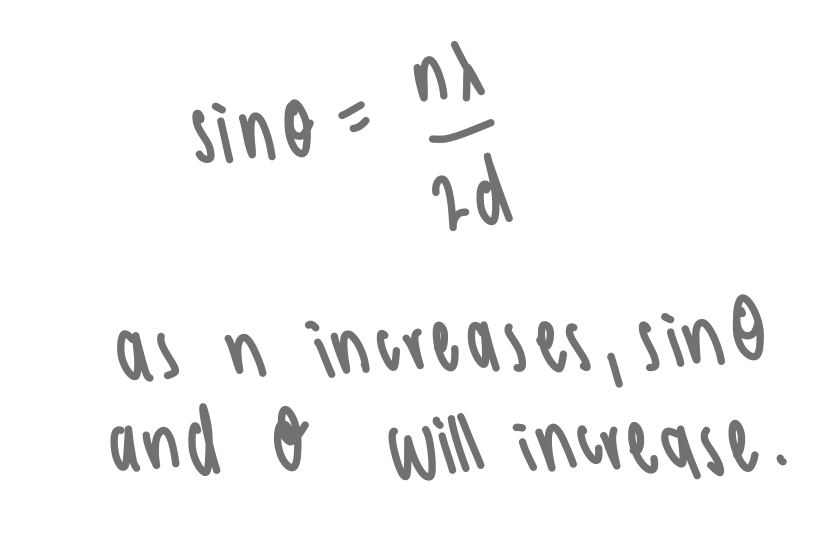
describe the reciprocal lattice
inverse relationship between crystal lattice and lattice of reflections (diffraction pattern)
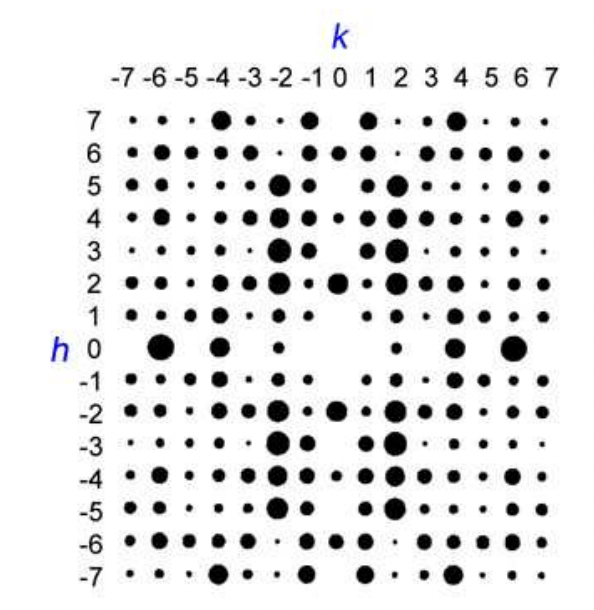
describe Miller indices
3 integers = h, k, l
= labels families of planes/points and reflection is corresponds to that give rise to diffraction spots due to constructive interference (also a label for the resulting reflection)
define the Laue equation *given*
used to determine d-spacing between planes/points of an given reflection
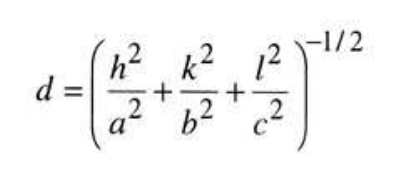
what is the unit angstrom?
A = x10^-10 m
what controls the position vs intensity of diffraction pattern
position = d spacing and Bragg angle = arrangement of unit cells:
crystal structure
intensity = extent of constructive interference = arrangement within unit cells:
unit cell structure
describe the application of diffraction spot positions
can be used to determine the Bragg angle = used to determine d
crystal symmetry
unit cell dimensions
estimare molecules in asymmetric unit
define the structure factor, F, and its amplitude, |F|
F = mathematical function describing the amplitude and phase of a wave diffracted from crystal lattice planes characterised by Miller indices h,k,l.
|F| = strength/intensity of diffracted wave
describe the application of diffraction spot intensities
I(hkl) = intensity of diffraction spot ∝ extent of constructive interference
F(hkl) = structure factor for given (hkl) indices
| F(hkl) | = amplitude of the wave scattered by one unit cell at (hkl) indices
larger amplitude = more constructive interference
smaller amplitude = less constructive interference
hence, amplitude of scattered wave ∝ intensity of diffraction spot
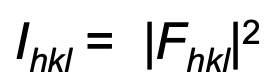
describes waves represented as vectors
vector = F
amplitude = |F|
direction = φ
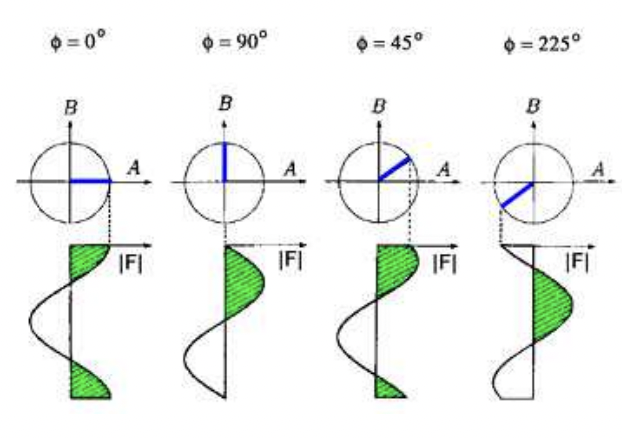
what equations define waves as vectors? *not given*
know the cos and sin
describe the phase problem
= phase information is lost during XRD
varying degrees of constructive interference between waves give rise to diffraction spots of varying intensities. the respective phases cannot be determined through the intensity of the diffraction spots.
there is no way to directly measure the phase (individual contribution to constructive interference) of the constituent waves
knowing the amplitude and phase of the initially scattered waves, how can the amplitude and phase of the final scattered wave be determined?
addition
find total A and total B
find total |F| and φ
describe structure factor vs structure factor amplitude
F = structure factor = complex
|F| = structure factor amplitude = not complex
describe atomic scattering factor
= measure of scattering power of an isolated atom = unique to an atom
= f(Φ)
depends on:
scattering amplitude of an isolated atom
Bragg angle of scattering
describe f(Φ) when Φ=0 (forward direction)
all electron scatter x-rays exactly in phase = no path difference
all scattered waves combine constructively
maximum scattering
describe f(Φ) when Φ>0
partial destructive interference increases as Φ increases
= intensity falls of as Φ increases
describe the effect of temperature on f
increased T = increased vibrations = spread out electron density = increases interference effects = scattering factor (f) falls off faster
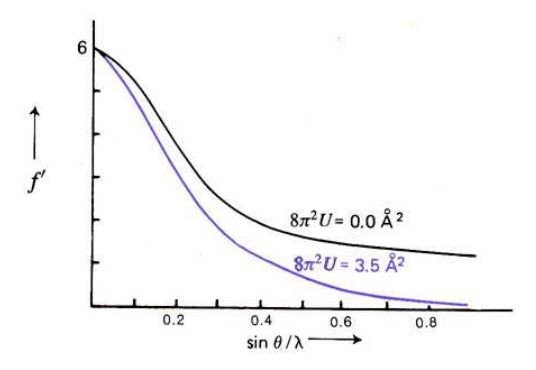
what is U?
= isotropic displacement parameter
= describes how much an atom deviates from its ideal position within a crystal lattice due to thermal vibrations
∝ 1/f
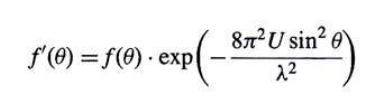
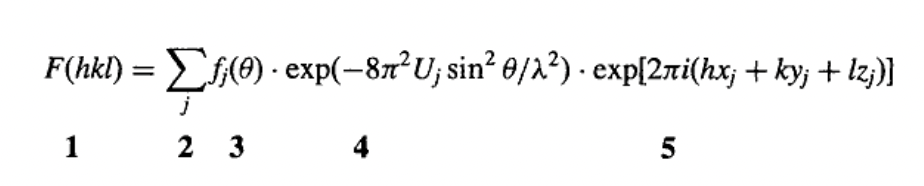
define the structure factor equation
2, 3, 4 = sum of the scattered x-rays
5 = scattered waves from each atom have different relative phases in direction hkl which depends on positions of atoms
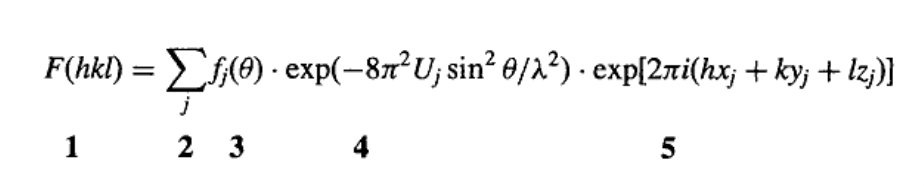
what is required to calculate structure factor, F?
chemical type (f)
position (xyz)
of each atom is known
describe the Fourier series
any periodic function can be described as a sum of simple series sin/cos functions
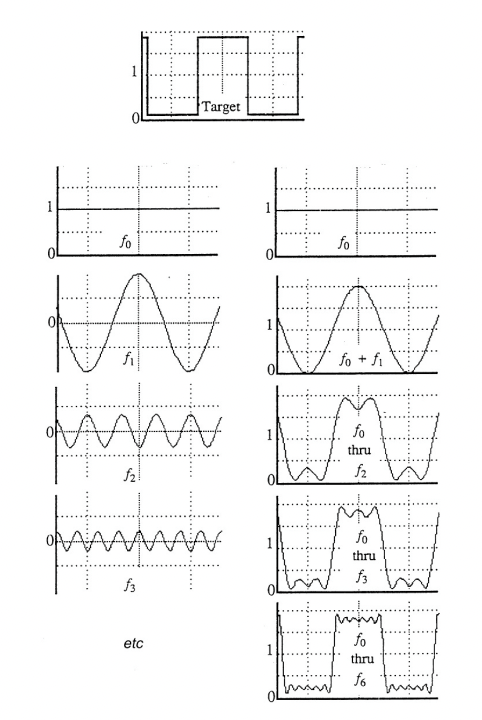
describe how the diffraction pattern is related to real space lattice
diffraction pattern → reciprocal lattice -(Fourier transform)→ real space lattice
what are methods of solving the phase problem
heavy atom method
direct method
describe the heavy atom method
substituting small number of atoms in the crystal with atoms of high atomic number which scatter X-rays more strongly and giving intense and detectable diffractions
diffraction pattern compared to normal diffraction patterns
Patterson function helps identify relative positions and phase information
describe the Patterson function
= Fourier transform of the squared amplitudes of structure factors F with all phases set to 0
= technique to determine electron density map purely on intensities of diffraction
= vector relationship
what is the height of Patterson peaks dependent on?
atomic numbers
often largest peak/atom is located at the origin
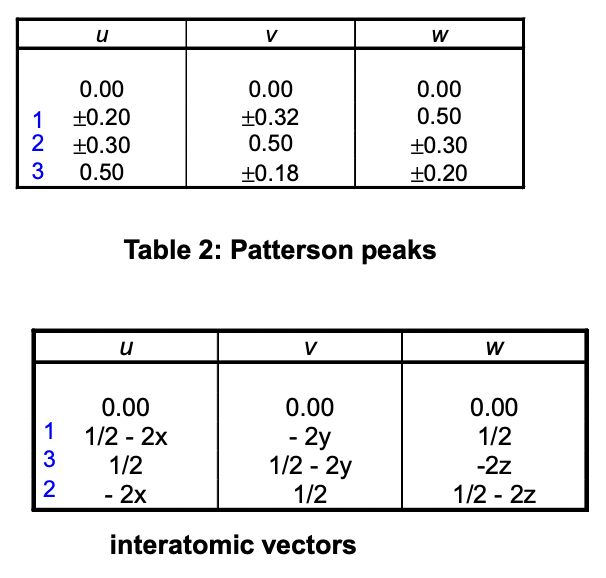
describe how interatomic vectors are found
subtracting the ‘central’ symmetry/origin from all the other symmetries
origin = simplest symmetry operator
calculate the interatomic vectors here
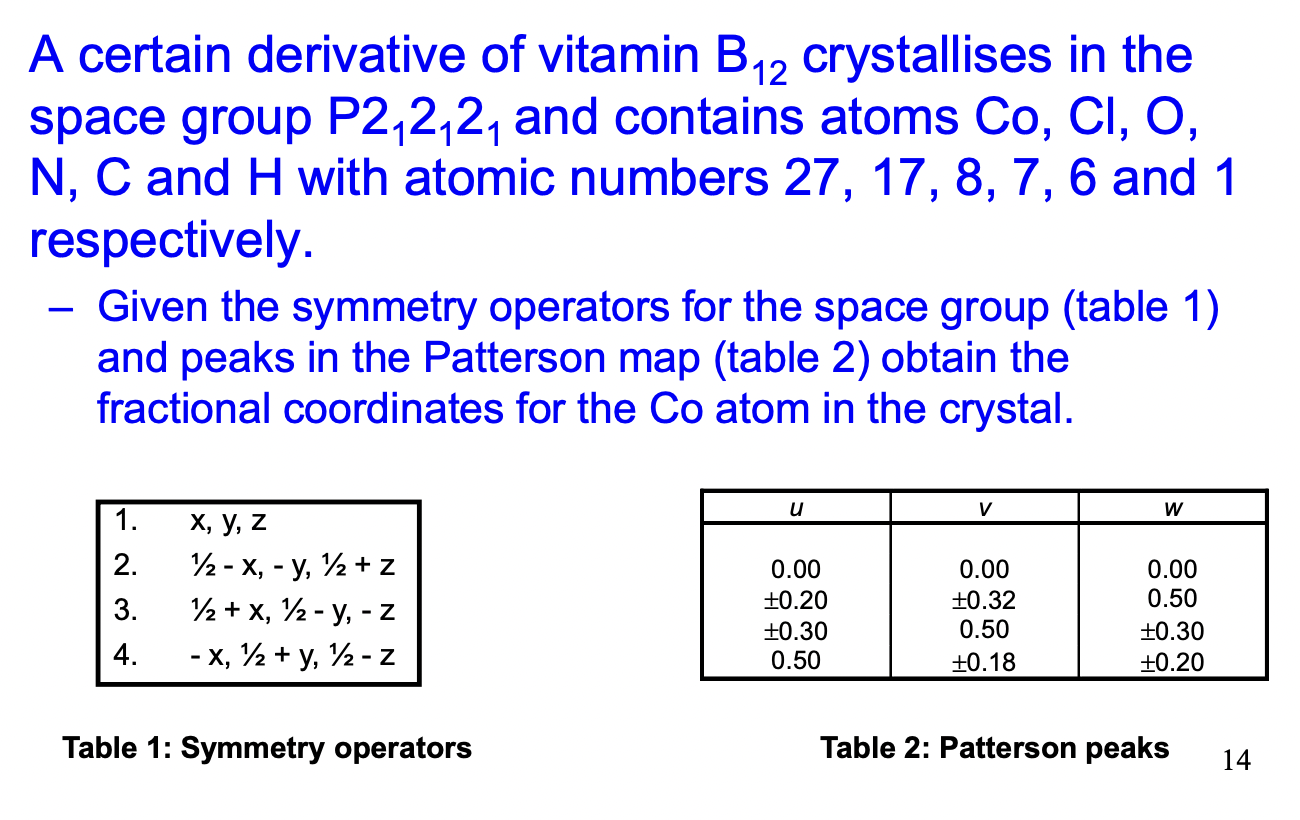
find interatomic vectors
match with patterson peaks (1/2 = 0.5)
rearrange to find x,y,z
x = 0.15
y = 0.16
z = 0.5
describe direct methods
guess then modify using probability and real-space constraints i.e. electron density must be +ve or 0
take the strongest E values only
phase signs are important = determine constructive/destructive interference
describe how the signs of the phases of reflection relate
products of the sign of two reflections is approximately equal to the sign of the sum of two reflections
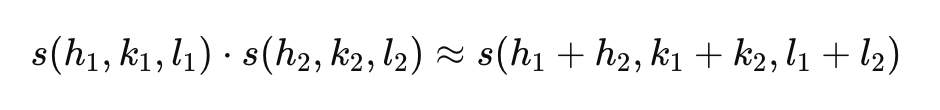
describe the application of heavy atom/direct methods for the structure factor calculation
provide (approximate) positions and chemical type
= obtain phase α(calc) from F(calc)
what are the two methods to find the structure factor
heavy atom/direct method → → F(calc) → → Fourier map
intensity of diffraction spots → → F(obs) → → Fourier map
list the necessary steps and calculations required to solve and then complete a single crystal X-ray structure
calculate P(uvw) (Patterson function/map) and find heavy atoms
calculate xyz of known atoms ^
compute |F(calc)| → α(calc) (approximate phases)
calculate p(xyz) from |F(obs)| and α(calc) -Fourier transform→ Fourier map
Fourier map → more atomic positions
refinement = step 2 and repeat
what are parameters of the refinement process?
R factor
difference maps
S
describe R-factors
R measures the agreement between observed structure amplitude and calculated (model) structure amplitude
small R = good model
large R = bad model
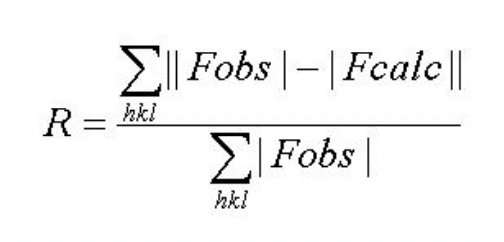
what is the application of Fourier maps
can be used to construct an electron density map
describe difference maps
∆p(xyz) = difference between the observed and calculated electron density at the point xyz
should be flat
+ve peak = atom omitted from model
-ve peak = wrong atom/atom position in model
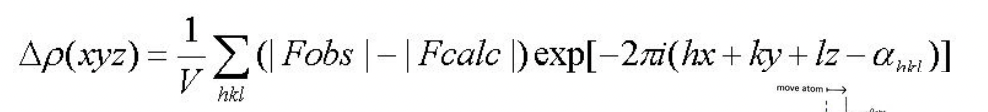
describe anisotropic temperature factors
anisotropic atomic motion can be described by 6 parameters which improve the fit of F(calc) with F(obs)
describe hydrogen atoms in electron density map
H = 1 e = weak scattering of X-rays
electron density shifted towards bond
often only discernible from difference map
describe the method of least squares
approach to refine models of best fit experimental data
S = residual sum of squares = quantities difference between obs and calc = want to minimise S
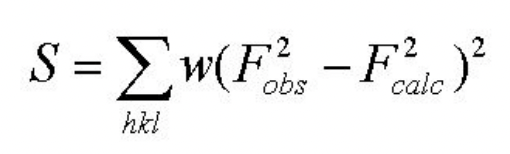
describe neutron diffraction
thermal neutrons have same wavelength as X-rays
X-rays = interaction with electron clouds (scattered by)
neutrons = interact with nuclei (scattered by)
diffraction pattern depends on:
nuclear scattering length = constant for a nucleus/isotope = +ve or -ve (constructive/destructive)
nuclear position
INDEPENDENT OF θ
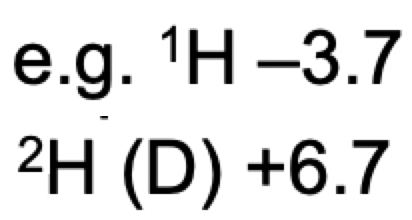
compare neutron scattering and x-ray scattering
xray scattering » neutron scattering
what is an application of neutron diffraction
distinguishing between isotopes in a structure
e.g. (1)H and (2)D
describe powder diffraction
combines diffraction patterns of a single crystal for all possible orientations of the crystal
= broad, diffuse pattern
also possible to resolve mixtures
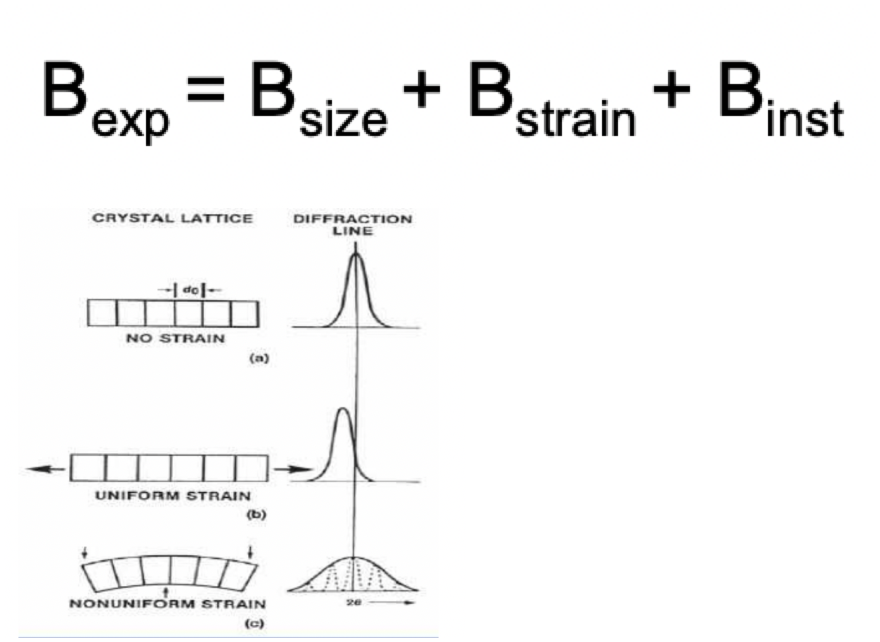
describe a diffraction peak profile
FWHM = full width at half maximum = B = measure of peak breadth
large B = small crystal
small B = large crystal
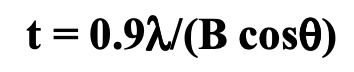
describe the different effects on B
inst = instrumental factors i.e. resolution
strain = distortions to crystal lattice
uniform strain: change in position of peak (uniform change in d/Bragg angle)
non-uniform strain: peak broadening (diffuse change in d/Bragg angle)
what are the different ‘levels’ of protein structure
primary = amino acid sequence
secondary = local polypeptide folding due to H bonding
tertiary = 3D folding of entire polypeptide chain due to interactions of R groups
what defines the absolute entropy of any object not given
w = number of possible states
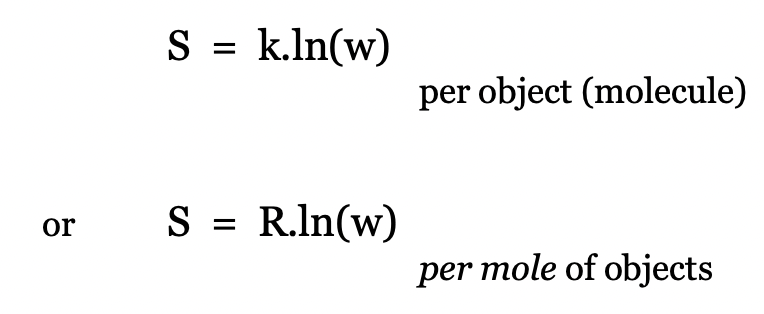
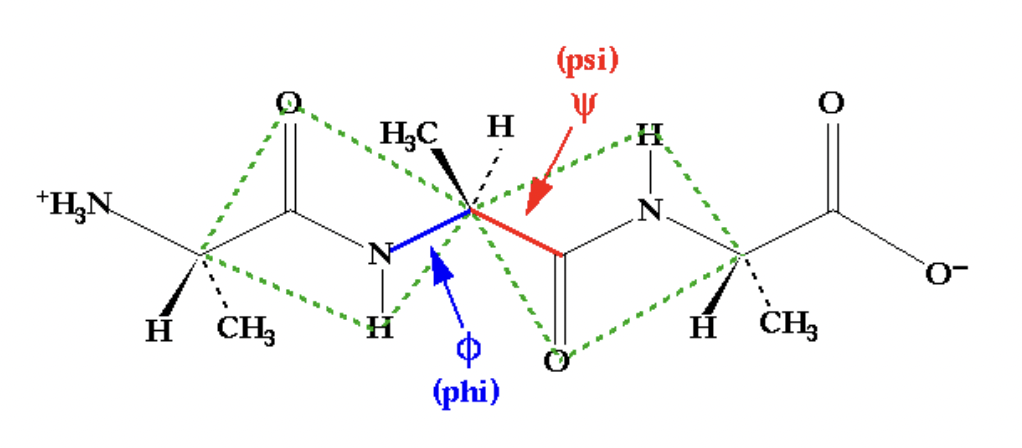
define the possible conformation of a peptide
psi ~ 3
theta ~ 3
hence 9 possible conformers for each peptide (ignoring side chains)
define the absolute entropy of peptide in a random coil
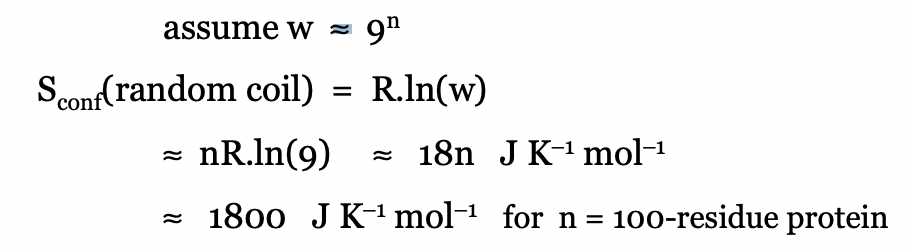
define the absolute entropy of peptide in native conformation
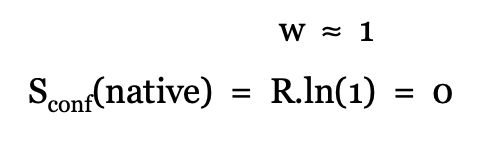
describe the free energy change of folding in terms of entropy
largely unfavourable
must be compensated for by H
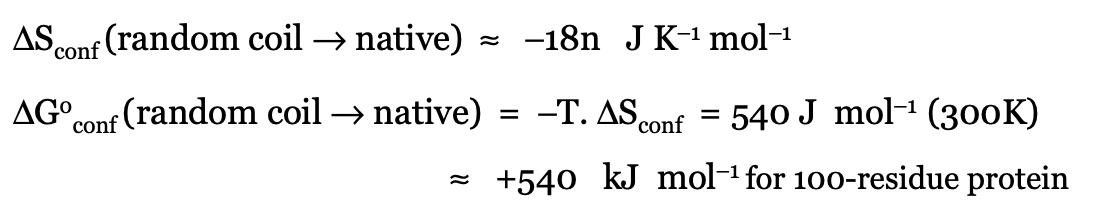
describe the directional folding of proteins
secondary structure = low energy conformation
molten globule structure = hydrophobic interactions
stabilisation of folded structure = directional H bonds
what are types of non-covalent interactions
electrostatic
H bonding (extreme electrostatic)
hydrophobic
what are types of electrostatic interaction (exc H bonding)
charge-charge
charge-dipole
dipole-dipole
dipole-induced dipole
transient dipole-induced dipole
describe the value of potential V
-ve = attractive
+ve = repulsive
define coulombs law
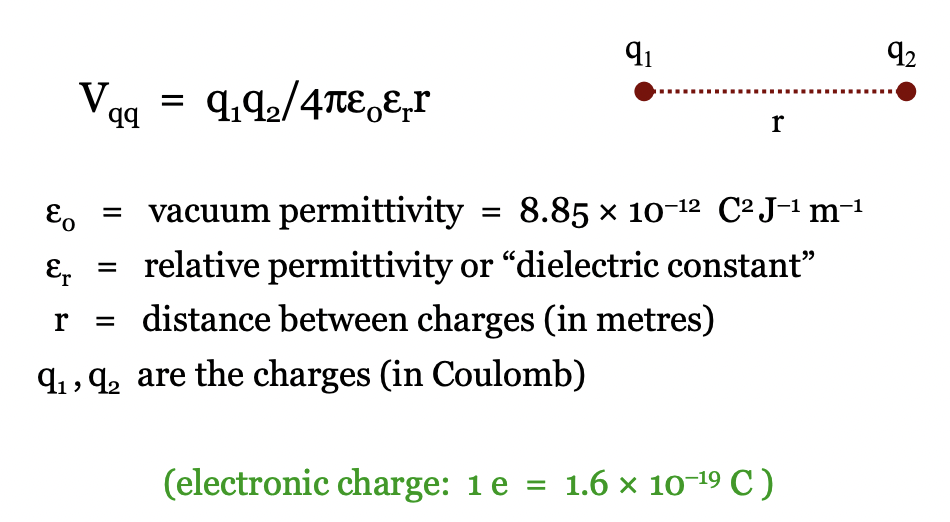
what can reduce V of coulomb interaction?
relative permittivity/dielectric constant
ionic screening
describe electrostatic interactions in vacuum vs water
water ««« vacuum
= ionic screening