14. Atomic Spectroscopy
1/12
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
13 Terms
(1.3c) T/F? One can add sodium chloride to a solution intended for elemental spectroscopic analysis in order to reduce contributions from ionization of the targeted metals.
True
(1.4b) What are refractory compounds, why are they a problem in elemental analysis, and what is the most effective way to fix the problem?
Refractory compounds are those that are prone to form oxides (e.g. Al, V, Si, Mg). The oxide form inhibits atomic absorption and emission, so it reduces the expected signal for a metal. The best way to reduce oxide formation is to use higher T. That is one reason ICP (7-9000K) is better than flame (2000K) for AA & Ae.
(3.3g) T/F? Metals with absorption lines <300 nm are likely detectable at lower concentrations using flame emission, rather than flame absorption, spectroscopy.
False; higher E transitions better probed by FAA bc more ground state atoms at a particular T
(3.3i) One area where FAA is more useful than ICP-AES, is for the qualitative determination of different metals in a sample.
False; ICP-OES better than flame AA for qualitative. Do not have to change lamps using ICP-OES.
(3.5a) Provide one common interference encountered in atomic spectroscopy, and tell how it can be corrected or minimized.
Any of the following:
1) Spectral - stray sources of light; residual molecular forms, unvaporized solvent droplets -> use higher T
2) Chemical - e.g. nonvolatile salts (PO43-) -> use a protecting agent like EDTA to bind metal or a releasing agent like La/Sr to bind phosphate
3) Oxide Formation - (refracting compounds) formed by Al, Ti, Mo, V -> use higher T to break
4) Ionization -> add NaCl as an ionization suppressant
5) Physical - sample uptake issues due to high viscosity, solids content; these affect flame T -> make sure you use matrix-matched standards for calibration
(3.5b) What limits the effectiveness of flame atomic absorption for qualitative analysis of the different metals in a sample?
In order to measure different metals by flame AA, you have to change the source lamp. Each lamp is made from and specific for the detection of a specific metal.
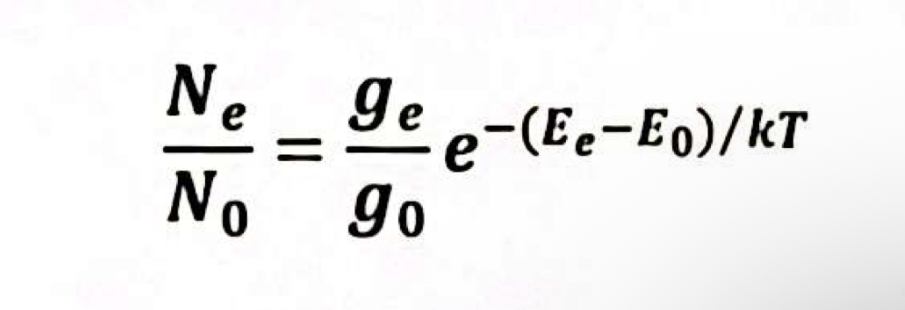
(4.5a) Define the variables in the equation below.
Ne = # excited state atoms
N0 = # ground state atoms
ge/g0 = statistical probabilities of excited and ground states
Ee/E0 = energies of excited and ground states
k = Boltzmann constant
T = temperature (kelvin)
(4.5b) What are the implications of this equation when you compare the use of a flame vs inductively-coupled plasma atomization source for atomic spectroscopy measurements?
The proportion of excited vs ground state atoms is determined by a Maxwell-Boltzmann distribution. As temperature is increased (during atomization in atomic spectroscopy), the proportion of atoms in the excited state will increase. Atomic emission experiments will be more sensitive if there are a greater number of excited state atoms, after atomization. Atomic absorption experiments are more sensitive when there is a greater proportion of ground state atoms.
ICP sources are high T (8000-10,000K); because this will create an abundance of excited state atoms for virtually all elements, atomic emission. Flame sources are lower T (3000K). At this T, some atoms (those with abs/emission lines <300nm, high E) will be better detected with absorption measurements (FAA) and others (abs/emission lines >300nm, low E) will be better detected by emission measurements (FAE).
(2.2+5.3) Why are ICP atomization sources used primarily for AE experiments, rather than AA experiments?
ICP sources have a very high T (8000-10,000K). Based on a Boltzmann distribution, higher T places more atoms into the excited state during atomization, especially relative to flame atomization sources, which operate at ~3000K.
Atomic emission experiments measure excited state atoms and they’re a much higher proportion of these in the high T source. In contrast, a flame source may be better for absorption experiments, because AA measures atoms in the ground state, and the lower temperature of the flame factors more ground state atoms.
(6.1) Why are flame-based atomic spectroscopy instruments available in atomic absorption (AA) and atomic emission (AE) formats, whereas inductively coupled plasma-based atomic spectroscopy instruments are available only in atomic emission (AE) format?

(6.5c) What are strategies to deal with chemical interferences, such as the presence of nonvolatile phosphates, in atomic spectroscopy measurements of metals?
Add a releasing agent, such as EDTA, which binds to the metals and displaces the phosphate.
Add a protecting agent, such as Sr, which binds to the phosphate and liberates the metal. Sr complexes readily with phosphate
What is the difference between FAA vs ICP-AES?
1) ICP-AES is excellent for qual/quant analysis while FAA is only quant
2) Temperature; Flame is ~3000K while ICP-AES is 8-10000K
3) LOD depends but GFAA is 10-1000x more sensitive
4) Linearity of FAA 1-3 orders bc of noisy flame while ICP-AES can do 7
5) Complexity and price on FAA is simpler and cheaper than ICP-AES
λ < 300 = _(1)_ is more sensitive
λ > 300 = _(2)_ is more sensitive
(1) absorption
(2) emission (but beware of interferences)