6 Shapes of molecules and IMF
1/59
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
60 Terms
what is electron repulsion theory
Pairs of electrons repel each other so will arrange themselves as far away as possible from each other
electron pairs surrounding a central atom therefore determine the shape of a molecule or ion
different numbers of electrons around an atom result in different shapes
amount of repulsion in pairs from greatest to least
Lone pair - lone pair (greatest)
lone pair -bonded pair
bond pair - bond pair (least)
why do lone pairs repel the most
lone pairs repel the most because they are slightly closer to the central atom therefore occupy more space
how much does a lone pair decrease the bond angle
2.5 degrees
if you have a multiple bond (e.g. double) does this count as more than one region of electrons
no
what is a molecule with 6 bonded pairs known as
octahedral
Name the shapes you need to know
linear
trigonal planar
tetrahedral
pyramidal
bent / non-linear
trigonal bipyramid
octahedral
why is methane tetrahedral
4 bonding regions
all bonding regions are bonded pairs
repulsion is equal
tetrahedral
why is ammonia pyramidal
4 bonding regions
1 lone pair
lone pair repels bond pairs more than bond pairs
pyramidal
why is water non-linear/bent
4 bonding regions
2 lone pairs that repel the most
followed by lone pair and bonded pairs which repel
least repulsion is between bond pair - bond pair
non linear/bent structure
What is a covalent bond
strong electrostatic bond between a shared pair of valence electrons and the nuclei of the bonded atoms
however electrons in a covalent bond aren’t always shared equally, some atoms are more electrically attractive than others
what is electronegativity and the name of the scale that measures it
measure of the attraction of a bonded atom for the pair of electrons in a covalent bond.
the Pauling scale
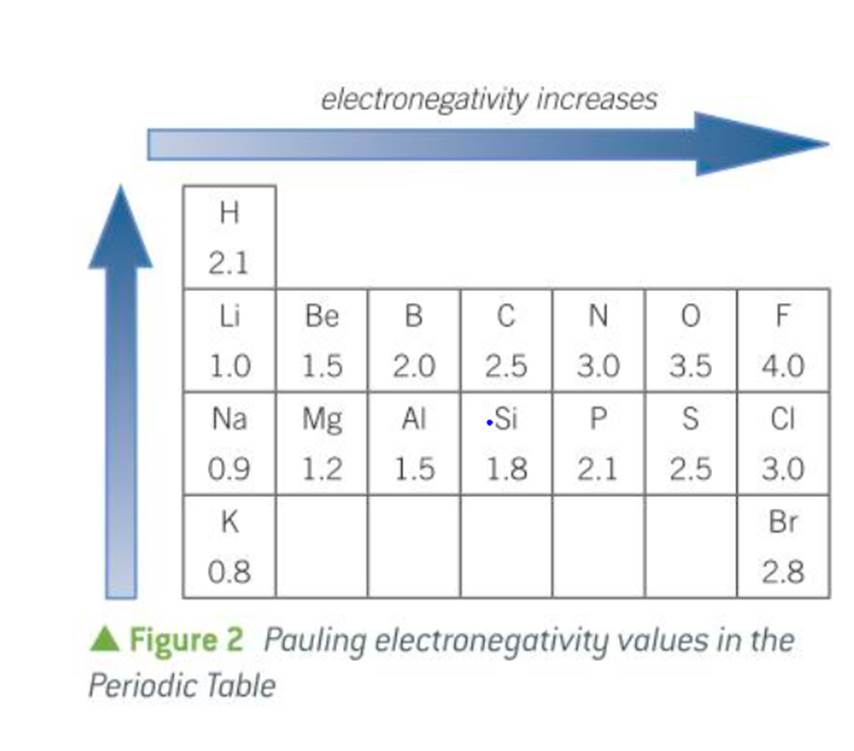
what is the link between bond type and electronegativity difference
covalent bonds have an electronegativity difference of 0
polar covalent bonds (e.g. HCl) have an electronegativity difference of 0 to 1.8
ionic bonds have an electronegativity difference greater than 1.8
this is why Beryllium Iodide is a covalent molecule despite having a metal and a non metal
factors than affect electronegativity
size of nuclear pull/charge (increases across a period as number of positive protons increases)
distance of electrons to pull of nucleus/atomic radius (decreases up group)
nonpolar covalent bond
Bonded electron pair is shared equally between bonded atom because the bonded atoms are the same or have similar/the same electronegativity
e.g. chlorine
all diatomic molecules are nonpolar (very limited/no electronegativity difference)
do nonpolar solvents mix with water
no - like dissolves like and water is polar
polar covalent bond
bonded electron pair is not shared equally between bonded atoms because they have different electronegativity
e.g. HCl
what is the polarity of a bond that has a permanent dipole
polar covalent bond is a permanent dipole
when is there an overall dipole
any shape NOT
trigonal planar or tetrahedral
why is water able to dissolve NaCl
water molecules are able to break down the ionic lattice due to the partially negative oxygen ions and the partially positive hydrogen ion.
water molecules attract Na+ and Cl- ion
the ionic lattice breaks down as it dissolves
in the resulting solution, water molecules surround the Na+ and Cl- ions
Na+ ions are attracted to the δ- oxygen of the water molecules
Cl- ions are attracted to the δ+ hydrogen of the water molecules
Permanent dipole dipole interaction
Name the 3 types of IMF
induced dipole dipole interactions (london forces)
permanent dipole dipole interactions
hydrogen bonding
what are IMF responsible for and what do covalent bonds determine
IMF are responsible for physical properties (mpt and bpt)
covalent bonds determine the identity and chemical reactions of molecules
what is the bond enthalpy for London forces
1-10
bond enthalpy for permanent dipole dipole interactions
3-25
bond enthalpy for hydrogen bonds
10-40
bond enthalpy for single covalent bonds
150-500
def London forces
London forces are weak IMF that exist between ALL molecules and atoms, whether polar or non-polar. They act between induced dipoles in different molecules.
how do London forces occur
movement of electron cloud produces a changing dipole in a molecule
at any instant, an instantaneous dipole will exist, but its position is constantly shifting
the instantaneous dipole induces a dipole on a neighbouring molecule
the induced dipole induces further dipoles on neighbouring molecules which attract one another
the MORE electrons in each molecule…
the larger the instantaneous and induced dipoles
the greater the induced dipole-dipole interactions
the stronger the attractive forces between molecules
more energy needed to overcome the IMF and separate molecules
higher bpt
larger numbers of electrons mean larger induced dipoles therefore greater induced dipole-dipole interactions therefore stronger attractive forces between molecules therefore more energy is needed to overcome the IMF and separate molecules therefore higher bpt
what molecules form permanent dipole interactions
polar molecules
the δ+ and δ- charges on polar molecules cause weak electrostatic forces of attraction between molecules.
these happen in ADDITION to (not instead of) London forces
def hydrogen bonds
a special type of permanent dipole interactions found between molecules containing
an electronegative atom with a lone pair of electrons (Oxygen, nitrogen, fluorine)
a hydrogen atom covalently bonded to an electronegative atom
why is solid ice less dense than water
hydrogen bonds hold water molecules in an open tetrahedral lattice structure with holes in it. this open structure decreases the density of water on freezing
when ice melts the ice lattice collapses and molecules move closer together, therefore the water molecules in ice are further apart than in water therefore less dense
why does water have a higher mpt and bpt than other simple molecules of similar size and mass?
each water molecules can form 4 hydrogen bonds
2 lone pairs on the oxygen
2 hydrogen atoms
this leads to a higher mpt and bpt as there are more IMF that need to be overcome for the molecules to be separated therefore more energy is needed
list of shapes u need to know
linear
trigonal planar
tetrahedral
pyramidal
bent/non-linear
trigonal bipyramid
octahedral
bond angle for linear
180
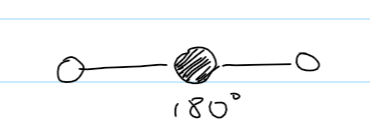
bond angle for trigonal planar
120
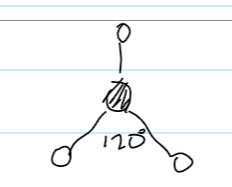
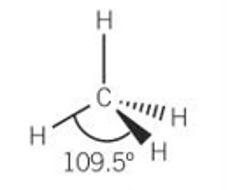
bond angle for tetrahedral
109.5
bond angle for pyramidal
107

bond angle for bent/non-linear
104.5

bond angles for trigonal bipyramid
120 and 90
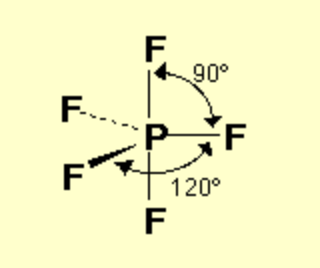
bond angle for octahedral
90
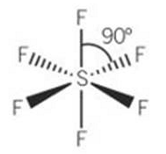
why is octahedral called octahedral
if the points are joined up it makes an octahedron
trigonal bipyramid diagram
example for linear molecule
CO2
example for trigonal planar
BF3
example for tetrahedral
CH4
example for pyramidal
ammonia NH3
example for bent/non-linear
water H2O
example for trigonal bipyramid
PF5
example for octahedral
SF6
funny molecules
S8
P4
most electronegative atom on pauling scale
fluorine
electronegativity difference for covalent bonds
0
electronegativity difference of polar covalent bonds
0-1.8
electronegativity difference for ionic bonds
greater than 1.8
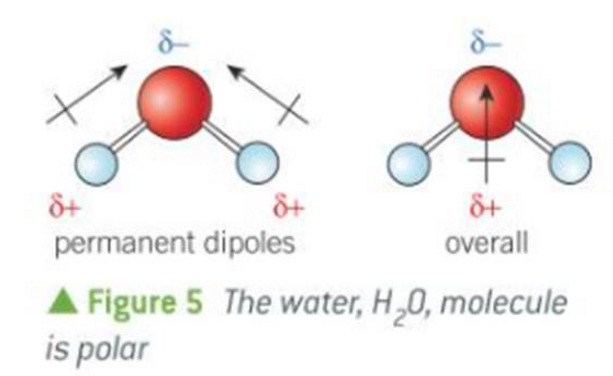
what does an overall dipole look like
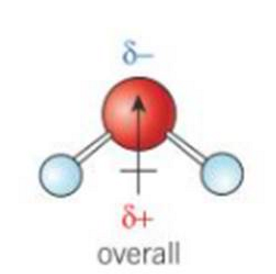
what do permanent dipoles look like
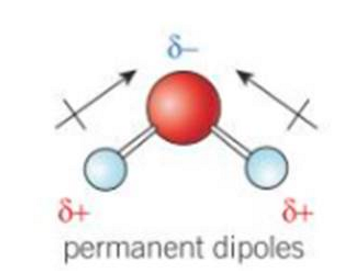
example of a polar molecule
water
when is there no overall dipole
when the dipoles cancel out
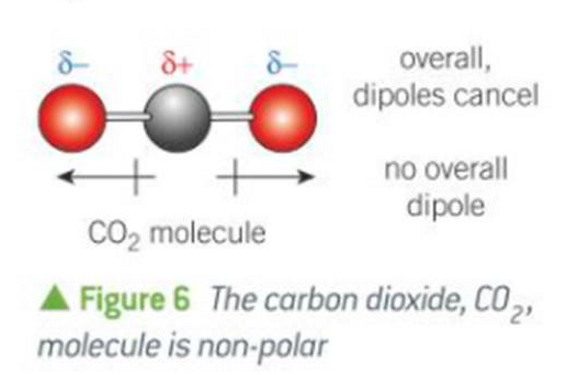
how to tell if a molecule is polar or non-polar
does it have symmetry?
tetrahedral shapes r only non-polar if the atoms on the ends are all the same
do the dipoles cancel out?
C and H are close enough in electronegativity that they cancel out