Instrumental Chem Exam 2 (conceptual)
1/60
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
61 Terms
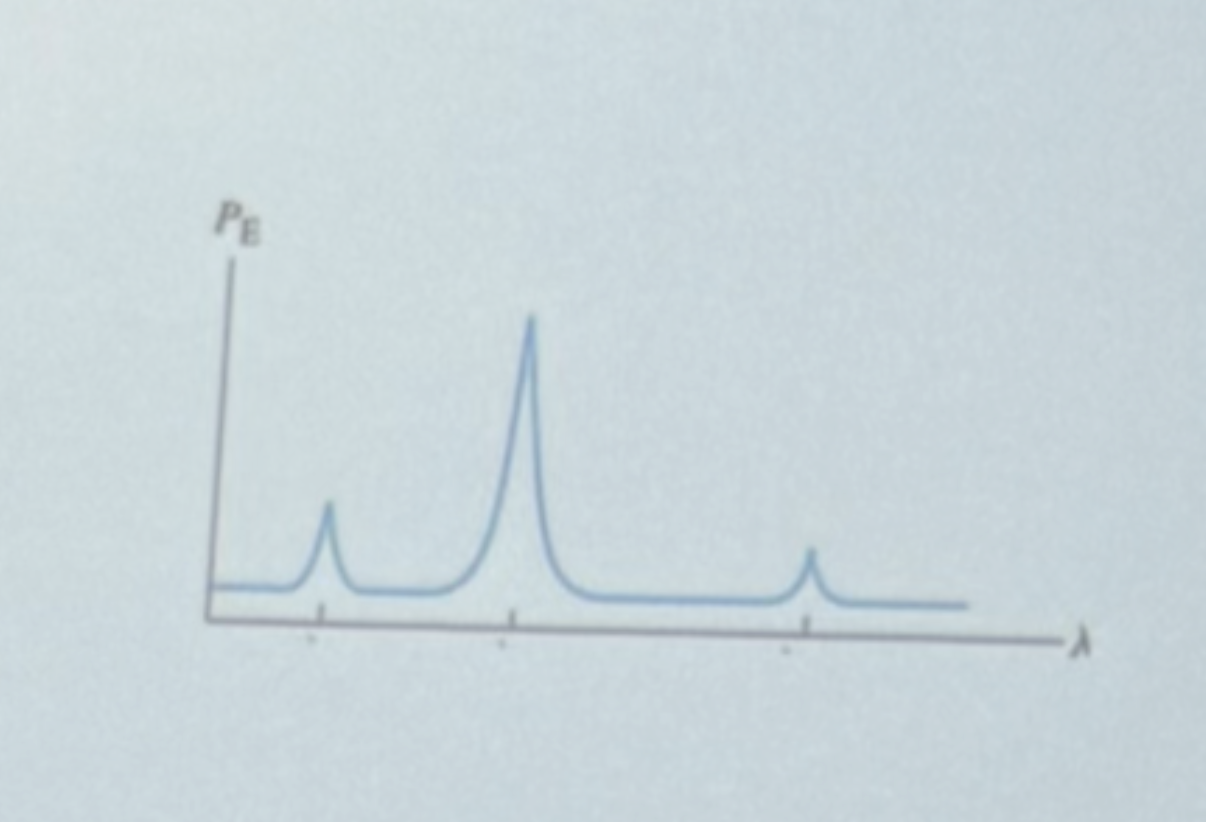
What electronic transition is responsible for the first peak (medium intensity, shortest wavelength)?
Lambda 2
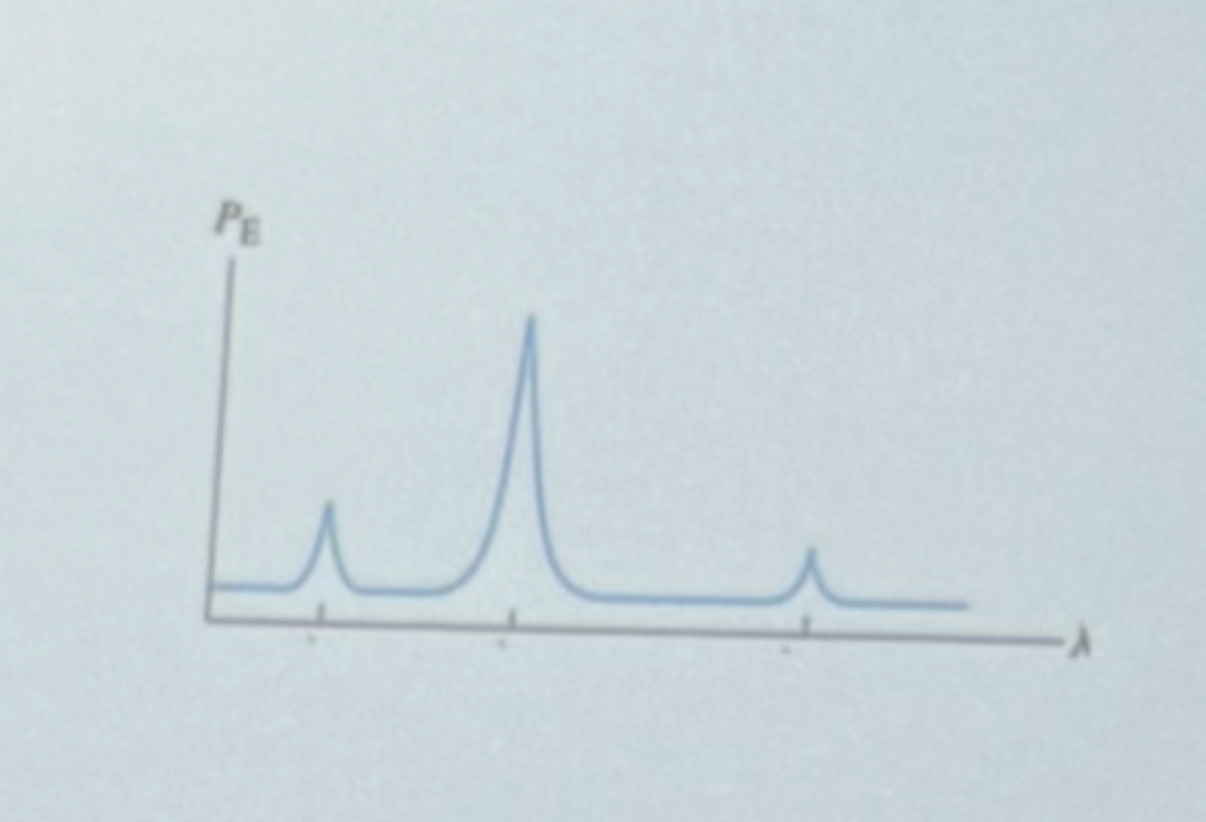
What electronic transition is responsible for the second peak (largest intensity, medium wavelength)?
Lambda 1
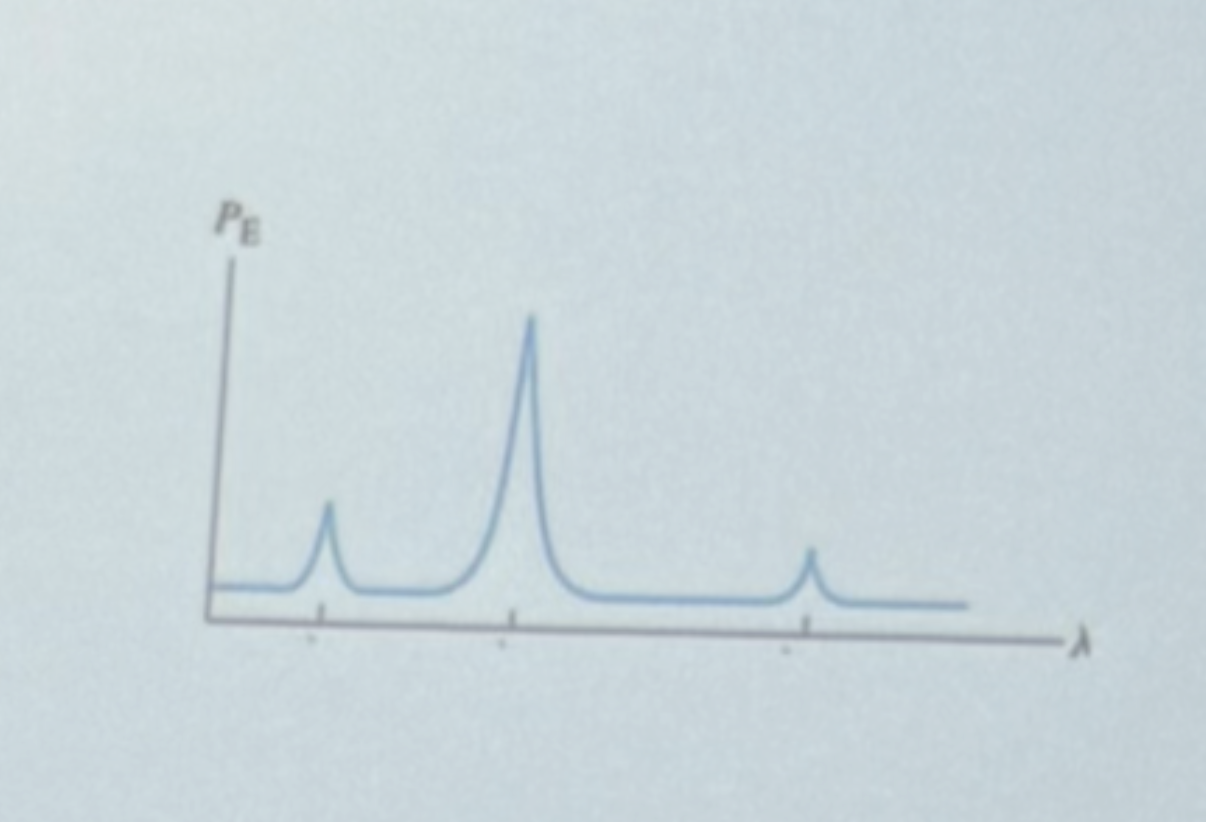
What electronic transition is responsible for the third peak (lowest intensity, longest wavelength)?
Lambda 21
What is fluorescence?
Emission of excited electrons that keep the original spin
What is phosphorescence?
A photoluminescence where the light lasts a long time
The intermolecular process in which a molecule crosses to a lower electronic state without emitting radiation is called
Internal conversion
The process in which a molecule in one spin state changes to another spin state with nearly the same total energy (eg singlet → triplet) is called
Intersystem crossing
A radiationless process in which a molecule loses electronic energy while transferring that energy to the solvent or another solute is called
External conversion
The main advantage of fluorescence over UV-vis absorption spectroscopy is
Its sensitivity
To collect a fluorescence emission spectrum
the excitation wavelength is held constant and the emission intensity is measured as a function of the emission wavelength
To collect a fluorescence excitation spectrum
The emission is measured at one wavelength while the exitation wavelengths are scanned
What is the correct representation for an orbital which has an “n” value of 4 and an “l” value of 2?
4d
How many orbitals in the d subshell
5
For a p sublevel, “l” equals ___
1
What is the correct representation for an orbital which has an “n” value of 3 and an “l” value of 1?
3p
For the quantum number, “l”, equal to 2, is an “ml” value of -1 permitted?
Yes
How many quantum numbers are needed to describe the energy state of an electron in an atom?
4
Is the following quantum numbers (n, l, ml, ms) possible? (4, 1, -1, +1/2)
Yes
In atomic absorption spectroscopy what types of sample introduction systems are used?
Both
When hydride generation is utilized for sample introduction, sodium borohydride (NaBH4) is often used to generate the volatile species of interest that is then swept into the absorption or emission spectrometer. What is the purpose of the borohydride?
It provides a source of hydride ions that react with the analyte to produce a volatile hydride (eg arsin gas, AsH3)
n analyzing solid samples which are of great historical significance, or which need to be preserved for other examinations, sample introduction techniques that are non-destructive or minimally disturb the sample are used. If a chemist was determining the trace element concentrations in the growth rings of trees to evaluate historical pollution paterns, what type of sample introduction method for atomic spectroscopy would be least destructive to the sample?
Laser ablation
A novice analyst needs to determine what trace elements are present in rubber samples that are used to make automotive tires. The laboriatory the analyst works in usually uses a spectrometer with an arc source (electrical sidchange) in the sample introduction system for their spectrometer. What problems is the analyst likely to encounter in analyzing these samples?
Rubber is an insulator, so in order to conduct electricity (and thus produce an arc discharge), it would have to be mixed with another conducting material.
The determination of the dissolved elements in natural water samples is normally completed by first filtering an aliquot of the sample to remove trace elements which are associated with particulate matter or large colloies. Sometimes, however, the total (dissolved plus particulate) concentration of dissolved elements is desired or filtering prior to anaysis is not practical. In this case, a laboratory that relies on a pneumatic nebulizer for sample introduction would be best served by using what type of nebulizer for analysis of total concentration of dissolved elements in natural water samples with some particulate content?
Babington
In inductively coupled plasma atomic emission spectroscopy (ICP-AES or just ICP), highly repredocible measreuments are made during the analysis of liquid samples because as the sample is introduced into the ICP-AES, the sample signal is scanned repeatedly and the collected signals are averaged. Which type of sample introduction system would be most suitable for ICP-AES?
Continuous
When analyzing samples with limited volumes, one would generally use an atomic absorption spectrometer equipped with a/an _____ atomizer
Either or
Almost all methods of analysis for elemental mercury rely on the absorbance of light at 254 nm by elemental mercury. In atomic absorption spectroscopy, when mercury is determined, what type of atmonization technique is normally used?
Cold vapor
Unlike other techniques which utilize absorption spectroscopy and have radiation sources that produce a continuum of radiation, in atomic absorption speectroscopy, the most common radiation sources are Hollow Cathode Lamps (HCLs) and Electrodeless Discharge Lamps (EDLs). These radiation sources produce _____ radiation which is characteristic of the element you wish to determine (and which is contained within the lamp you are using).
Discrete
The higher pressures and temperatures in the Hollow Cathode Lamp (HCL) relative to the temperatures in the flame allow for absorptions by the analyte to occur within the narrower bandwidth of an HCL emission.
False
Most commercially abailable atomic absorption spectrophotometers utilize a double-beam spectrometer in which the source radiation is passed intermittently through and then around the flame. The purpose of this is to be sure that the background signal that is recorded includes any light radiation that is emitted by the dflame itself and most importantly to determine the radiation emitted power (Pr) of the lamp itself. In the typical double-beam spectrophotometer used in AAS, a _______ is used to alternatively direct radiation from the source through the flame and then around the flame, repeatedly.
Chopper
Unlike inductively coupled plasma techniques, atomic absorption spectroscopy is subject to a number of chemical interferences. One of these interferences occurs when chemical compounds in the sample form compounds in the atomized sample that do not readily absorb light that is characteristic of the element of interest. One type of agent that can be used to reduce chemical interferences is called a ______ agent.
Releasing
Why is an electrothermal atomizer more sensitive than a flame atomizer?
The average residence time of analyte in the optical path is several seconds
Atoms are in constant motion. What is the effect of this motion on the wavelength(s) observed by the dettector when these molecules emit radiation?
Atoms moving toward the detector will have shorter wavelengths, atoms moving away will have longer wavelengths
When the wavelength of emitted radiation is set to 300 nm, what happens to the width of the line when the lifetime of the excited state increases?
Becomes more narrow
Why are ionization interferences less severe in ICP than in flame emission spectroscopy?
The plasma contains a high concentration of electrons
Which of the following components make use of a thin metal foil to isolate a nearly monoenergetic excitation beam?
Filters
Energy dispersive system uses which of the following detectors?
Semiconductor detector
In energy dispersive systems, the energy level of pulses is related to which of the following?
Element involved
The analysis of x-ray beam by diffraction is similar to spectrum analysis carried out with a diffraction grating.
True
The crystal used as x-ray grating has ___ dimensional lattice arrays?
3
Which of the following can be done to avoid loss of intensities of x-rays due to absorption of long wavelength x-rays?
Air in the chamber must be replaced by helium
Which of the following is the disadvantage of silicon semiconductor detector?
Can be operated only at low temperatures
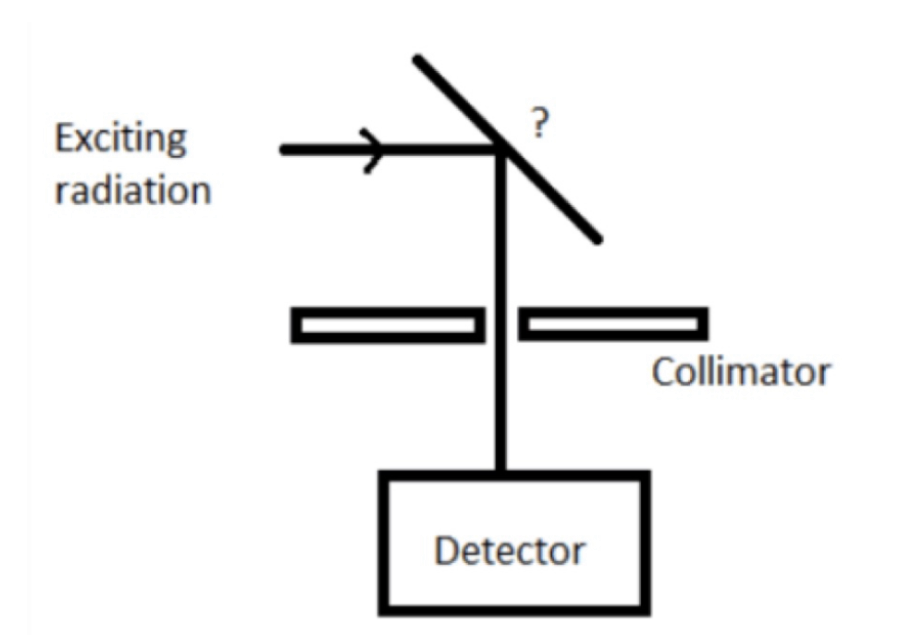
Given is the diagram of energy dispersive system. Identify the unmarked component.
Specimen
Sulfer dioxide is a nonlinear molecule. How many vibrational modes will this compound have?
3
Sulfer dioxide is a nonlinear molecule. How many IR absorption bands would sulfer dioxide be expected to have?
3
Indicate whether the following vibrations are active or inactive in the IR spectrum: (a) CH3-CH3 C-C stretching
Inactive
Indicate whether the following vibrations are active or inactive in the IR spectrum: (b) CH3-CCl3 C-C stretching
Active
Indicate whether the following vibrations are active or inactive in the IR spectrum: (a) SO2 Symmetric stretching
Active
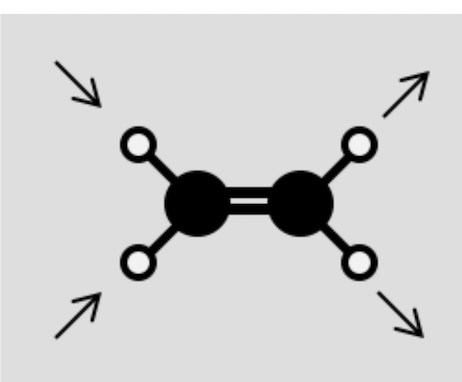
Indicate whether the following vibrations are active or inactive in the IR spectrum: (d) CH2=CH2 C-H stretching
Active
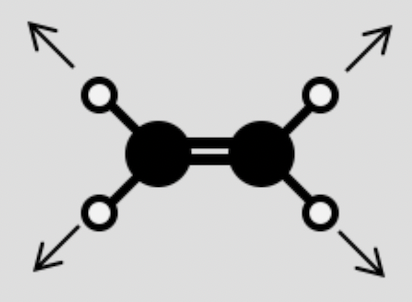
Indicate whether the following vibrations are active or inactive in the IR spectrum: (e) CH2=CH2 C-H stretching
Inactive
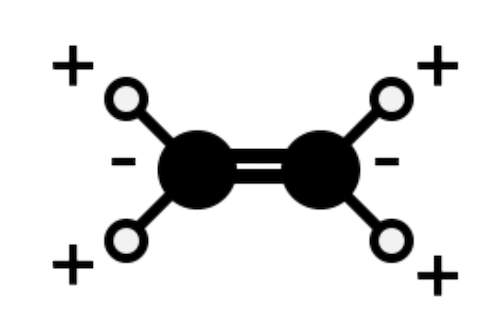
Indicate whether the following vibrations are active or inactive in the IR spectrum: (f) CH2=CH2 CH wag
Active
Identify the following vibration
Asymmetric stretching
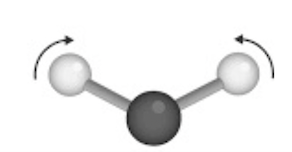
Identify the following vibration
In-plane scissoring
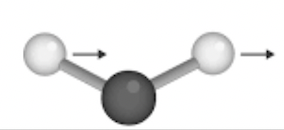
Identify the following vibration
In-plane rocking
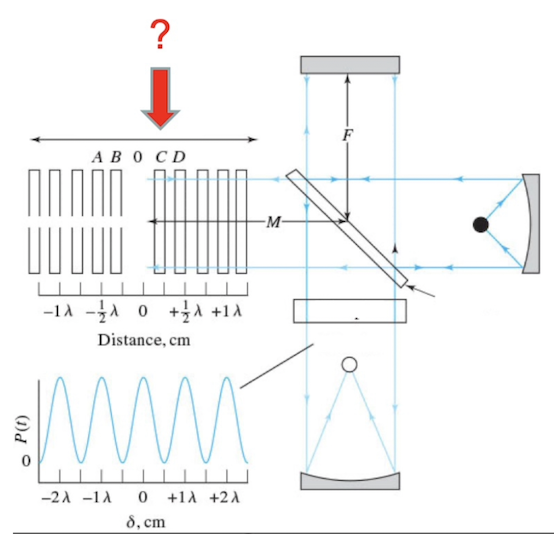
Identify the following component of a Michelson interferometer
Movable mirror
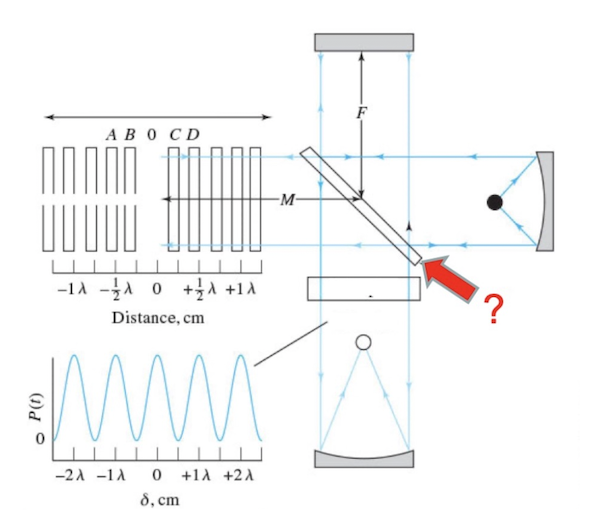
Identify the following component of a Michelson interferometer
Beamsplitter
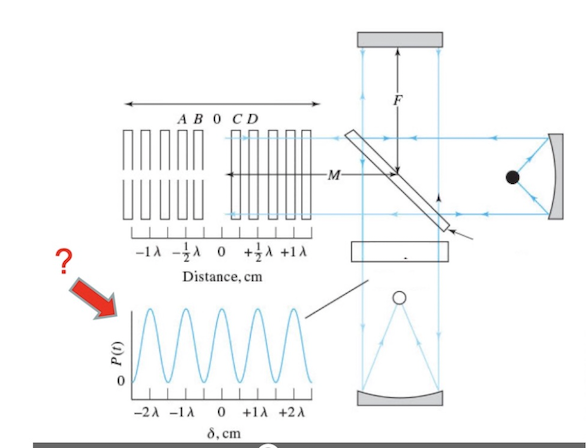
Identify the following component of a Michelson interferometerI
Interferogram
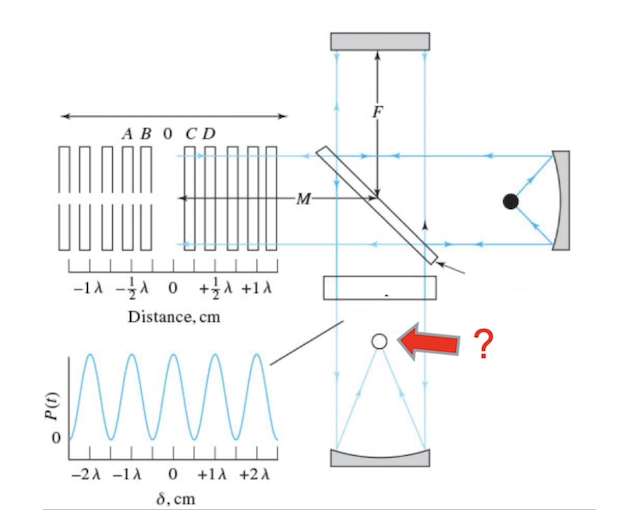
Identify the following component of a Michelson interferometer
Detector
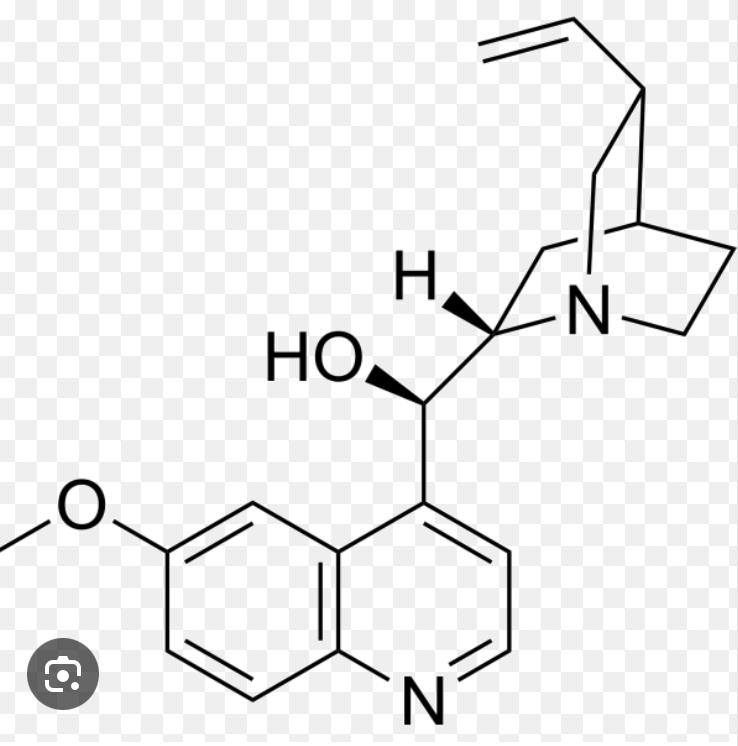
Quinine is one of the best known fluorescent molecules, and the sensitivities of fluorometers are often specified in terms of the detection limit for this molecule. The structure of quinine is given next. Predict the part of the molecule that is most likely to behave as the chromophore and fluorescent center.
Left double ring
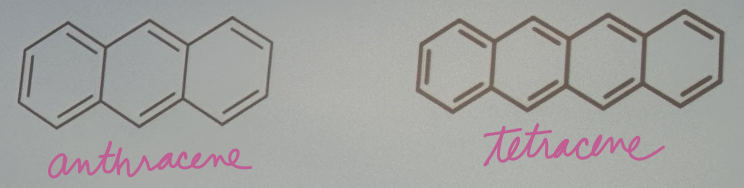
For this question, consider anthracene and tetracene. (a) which molecule would you expect to have a larger quantum yield?
Tetracene
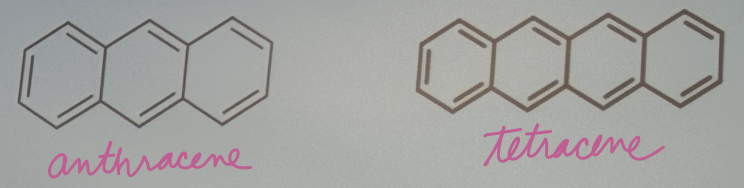
For this question, consider anthracene and tetracene. (b) which molecule would you expect to have a shorter peak wavelength for fluorescence?
Tetracene
For the same concentration of Ni, the absorbance of 352.4 nm was found to be about 30% greater for a solution that contained 50% ethanol than for an aquarium solution. Explain
Creating a fine mist