Exam Review Practice Questions
1/19
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
20 Terms
Give the definitions of the following changes of state: evaporation, boiling, sublimation, melting, freezing, deposition, condensation.
1. Evaporation:
Definition: The process in which a liquid turns into vapor at a temperature below its boiling point.
Example: Water evaporating from a puddle on a sunny day.
2. Boiling:
Definition: The rapid vaporization of a liquid, typically when heated to its boiling point.
Example: Water boiling in a pot on a stove.
3. Sublimation:
Definition: The transition of a substance directly from the solid phase to the gas phase without passing through the intermediate liquid phase.
Example: Dry ice (solid carbon dioxide) sublimating into carbon dioxide gas.
4. Melting:
Definition: The process in which a substance changes from a solid to a liquid state as it is heated.
Example: Ice melting into water when exposed to heat.
5. Freezing:
Definition: The process in which a substance changes from a liquid to a solid state as it loses heat.
Example: Water freezing into ice in a freezer.
6. Deposition:
Definition: The phase transition in which a gas transforms directly into a solid without passing through the liquid phase.
Example: Frost forming on a cold surface.
7. Condensation:
Definition: The process in which a gas or vapor changes into a liquid state as it loses heat.
Example: Water vapor in the air condensing into liquid water droplets on a cold surface.
Identify differences between solids, liquids, and gases
1. Particle Arrangement:
- Solids: In a solid, particles are closely packed together in a fixed and orderly arrangement. The particles vibrate but do not move from their fixed positions.
- Liquids: In a liquid, particles are close together but not in a fixed position. They can move past one another, allowing liquids to flow and take the shape of their container.
- Gases: In a gas, particles are far apart and have no fixed arrangement. Gases completely fill the available space and expand to fill the shape of their container.
2. Volume and Shape:
- Solids: Have a definite shape and volume. The shape is rigid and does not change easily.
- Liquids: Have a definite volume but take the shape of their container. They flow and are not rigid in shape.
- Gases: Take the shape and volume of their container. They have neither a definite shape nor a definite volume.
3. Density:
- Solids: Generally have a higher density compared to liquids and gases due to the close packing of particles.
- Liquids: Have a higher density than gases but lower density than solids.
- Gases: Have the lowest density as the particles are spread out and have more kinetic energy.
4. Compressibility:
- Solids: Generally not compressible, as the particles are already closely packed.
- Liquids: Not very compressible, but more compressible than solids.
- Gases: Highly compressible because the particles are far apart, allowing them to be squeezed closer together.
5. Kinetic Energy:
- Solids: Particles have low kinetic energy and mostly vibrate in fixed positions.
- Liquids: Particles have higher kinetic energy than solids, allowing them to move and flow.
- Gases: Particles have the highest kinetic energy and move freely in all directions.
6. Melting and Boiling Points:
- Solids: Have a specific melting point at which they change from solid to liquid.
- Liquids: Have a specific boiling point at which they change from liquid to gas.
- Gases: Do not have a fixed boiling point; they turn into liquids when cooled and solids when further cooled.
For each of the following, change the following temperatures given in Celsius to absolute temperature
in Kelvin
a) 0°C b) 25°C c) 58°C d) -40°C e) -112°C
K=°C+273.15
In this formula:
K represents the temperature in Kelvin.
°C represents the temperature in Celsius.
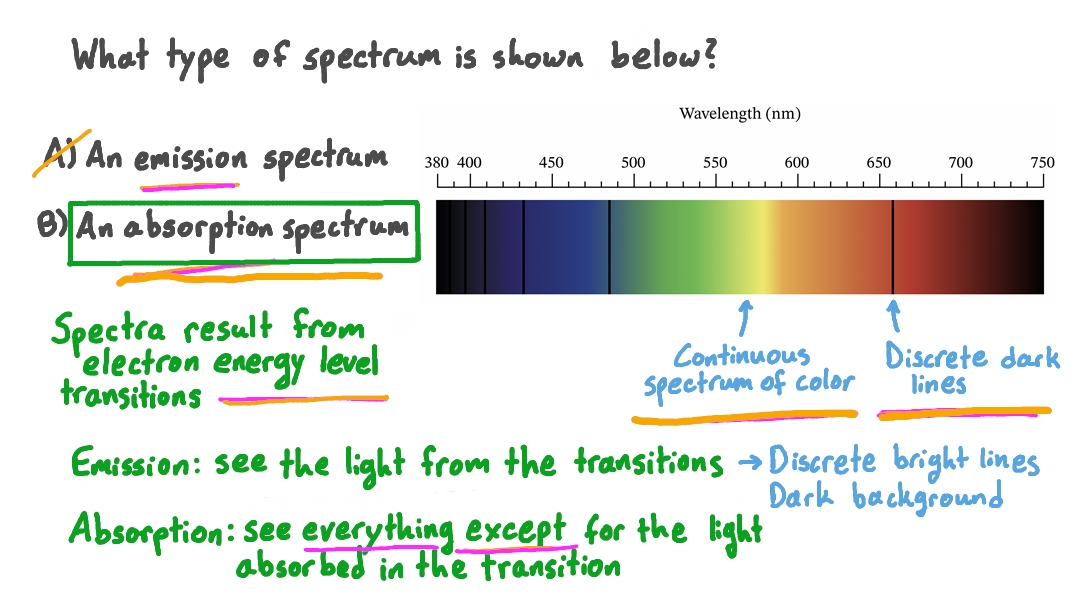
Distinguish between an absorption spectrum of an element and an emission spectrum of an element.
Include a picture of each.
Absorption spectrum:
Continuous spectrum of light.
Emission spectrum:
Bright lines dark background.
1. Absorption Spectrum:
- Definition: An absorption spectrum is formed when atoms absorb certain wavelengths of light as electrons transition from lower energy levels to higher energy levels.
- Process: When light passes through a sample of atoms, specific wavelengths are absorbed by the electrons in the atoms, causing them to move to higher energy levels.
- Characteristics: The absorption spectrum appears as dark lines or bands (absorption lines) at the wavelengths corresponding to the energies needed for electronic transitions.
- Nature of Lines: Absorption lines appear on a continuous spectrum background, indicating the wavelengths that have been absorbed.
2. Emission Spectrum:
- Definition: An emission spectrum is formed when atoms emit light at certain wavelengths as electrons transition from higher energy levels to lower energy levels.
- Process: When atoms are excited (e.g., by heat or an electric field), electrons move to higher energy levels. As they return to lower energy levels, they release energy in the form of light.
- Characteristics: The emission spectrum appears as bright lines or bands (emission lines) at the wavelengths corresponding to the energies released during electronic transitions.
- Nature of Lines: Emission lines appear on a dark background, indicating the specific wavelengths at which light is emitted.
3. Source of Light:
- Absorption Spectrum: The light source is usually a continuous spectrum, and the absorption lines indicate the wavelengths that have been absorbed by the atoms.
- Emission Spectrum: The light source is often the excited atoms themselves emitting light at specific wavelengths.
4. Temperature Influence:
- Absorption Spectrum: Can be observed at any temperature when light passes through a sample of atoms.
- Emission Spectrum: Typically observed at elevated temperatures when atoms are excited and emit light.
5. Observation:
- Absorption Spectrum: Observed when light passes through a cool and dilute gas or a sample containing the atoms of the element.
- Emission Spectrum: Observed when the atoms of an element are in an excited state, leading to the emission of light.
In summary, absorption spectra involve the absorption of specific wavelengths of light, resulting in dark lines on a continuous spectrum, while emission spectra involve the emission of light at specific wavelengths, resulting in bright lines on a dark background.
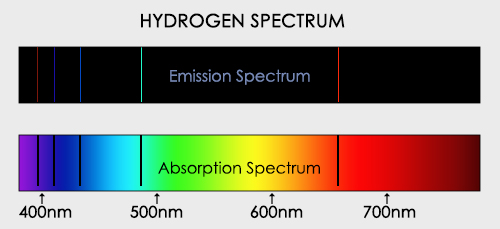
Define ionization energy. Why does it increase as one moves from left to right across a period? Why does it decrease as one moves down a group?
Ionization Energy:
Ionization energy is the energy required to remove an electron from an atom in its gaseous state. It is typically expressed in units of energy per mole, such as kilojoules per mole (kJ/mol) or electron volts (eV).
Trends in Ionization Energy:
1. Period.
Increase from Left to Right Across a Period:
- As you move from left to right across a period (from alkali metals to noble gases) in the periodic table:
Atomic Size:
Generally decreases because the electrons are added to the same energy level while the nuclear charge increases.
Effective Nuclear Charge:
Increases due to the increasing number of protons in the nucleus. More protons greater pull on the electrons resulting in a greater ionization energy.
Electron-Electron Repulsion:
Remains relatively constant within a shell.
Trend in Ionization Energy:
Increases because it becomes more difficult to remove an electron from a smaller, more tightly bound atom with a higher effective nuclear charge.
2. Group
Decrease Down a Group:
- As you move down a group (from top to bottom) in the periodic table:
Atomic Size:
Generally increases due to the addition of new energy levels.
Effective Nuclear Charge:
Increases slightly but is offset by the increased distance between the outer electrons and the nucleus.
Electron-Electron Repulsion:
Increases due to the addition of electron shells, but this effect is outweighed by the other factors.
Trend in Ionization Energy:
Decreases because electrons are farther from the nucleus, making them easier to remove. The outer electrons are shielded by inner electron shells, reducing the effective nuclear charge experienced by the outermost electrons.
In summary, ionization energy increases from left to right across a period due to the decreasing atomic size and increasing effective nuclear charge. Conversely, ionization energy decreases down a group due to the increasing atomic size and decreasing effective nuclear charge, making it easier to remove electrons from outer energy levels.
Sketch what a graph of successive ionization energies of the following elements would look like.
How would one go about this? Better yet how would one ascribe specific numerical values to the energy values for each of the electrons?
Just understand general trend.
Structure 1.3: Electron Configurations
6. If it takes 3.36 x 10-19 J of energy to eject an electron from the surface of a certain metal, calculate the longest possible wavelength of light that can ionize the metal.
7. A certain green light has a frequency of 6.26 x 1014 s-1. What is the wavelength of this light? What is the energy of one photon of this light?
8. The ionization energy of gold is 890.1 kJ/mol. Calculate the minimum wavelength of light that will ionize gold.
What assumptions are made about how ideal gases behave?
Structure 1.5: Ideal Gases
1. Particles have negligible volume:
- The volume occupied by the gas particles themselves is assumed to be insignificant compared to the total volume of the gas. In other words, gas particles are treated as point masses.
2. No intermolecular forces:
- Ideal gases are assumed to have no attractive or repulsive forces between particles. This means that there are no intermolecular forces of attraction or repulsion operating between gas molecules.
3. Constant, random motion:
- Gas particles are assumed to be in constant, random motion. They move in straight lines until they collide with each other or the walls of the container.
4. Elastic collisions:
- Collisions between gas particles and between particles and the walls of the container are assumed to be perfectly elastic. This means that there is no loss of kinetic energy during collisions.
5. Average kinetic energy is proportional to temperature:
- The average kinetic energy of gas particles is directly proportional to the temperature of the gas in kelvin. This is described by the equation KE=(1/2) mv^2, where \( m \) is the mass of a gas particle and \( v \) is its velocity.
6. Pressure is due to collisions:
- The pressure exerted by a gas is a result of the collisions of gas particles with each other and with the walls of the container. More collisions result in higher pressure.
7. No energy transfer during collisions:
- While collisions between gas particles result in energy transfer, there is no net transfer of energy between the particles during collisions. The total kinetic energy of the system remains constant.
Explain the following observations for ionic compounds
a) Higher ion charges result in higher melting points
b) Larger ionic radii result in lower melting points
c) They are non-conductive as solids but conductive in the molten state or aqueous state
a) Higher Ion Charges Result in Higher Melting Points:
- Explanation: The strength of the electrostatic forces holding ions together in an ionic compound is directly influenced by the magnitude of the ion charges. Higher ion charges result in stronger electrostatic attractions between the positively and negatively charged ions in the crystal lattice.
- Effect on Melting Points: Stronger attractions require more energy to overcome, leading to higher melting points. Therefore, ionic compounds with higher charges on their ions tend to have higher melting points.
b) Larger Ionic Radii Result in Lower Melting Points:
- Explanation: The size of the ions also affects the strength of the electrostatic forces in an ionic compound. Larger ions have a more diffuse electron cloud, leading to weaker attractions between ions.
- Effect on Melting Points: Weaker attractions are easier to overcome, requiring less energy to break the bonds and transition from a solid to a liquid state. Thus, ionic compounds with larger ionic radii generally have lower melting points.
c) Non-conductive as Solids, but Conductive in the Molten State or Aqueous State:
- Explanation: In the solid state, ions in an ionic compound are held in a fixed position within the crystal lattice, and they are not free to move. As a result, ionic compounds are non-conductive as solids.
- In the Molten State or Aqueous State: When an ionic compound is melted or dissolved in water, the ions become mobile. In the molten state or aqueous solution, the ions can move freely and carry an electric current.
- Effect on Conductivity: Conductivity in the molten state or aqueous solution is a result of the movement of charged particles (ions), allowing them to carry an electric current. The ability to conduct electricity is a distinctive feature of ionic compounds when they are in a state where ions can move.
In summary, the properties of ionic compounds, including melting points and conductivity, are influenced by the strength of electrostatic forces between ions. Higher ion charges and smaller ionic radii result in stronger attractions, leading to higher melting points. Ionic compounds are non-conductive as solids due to the immobility of ions, but they become conductive in the molten state or aqueous solution when ions are free to move.
VSEPR DIAGRAMS
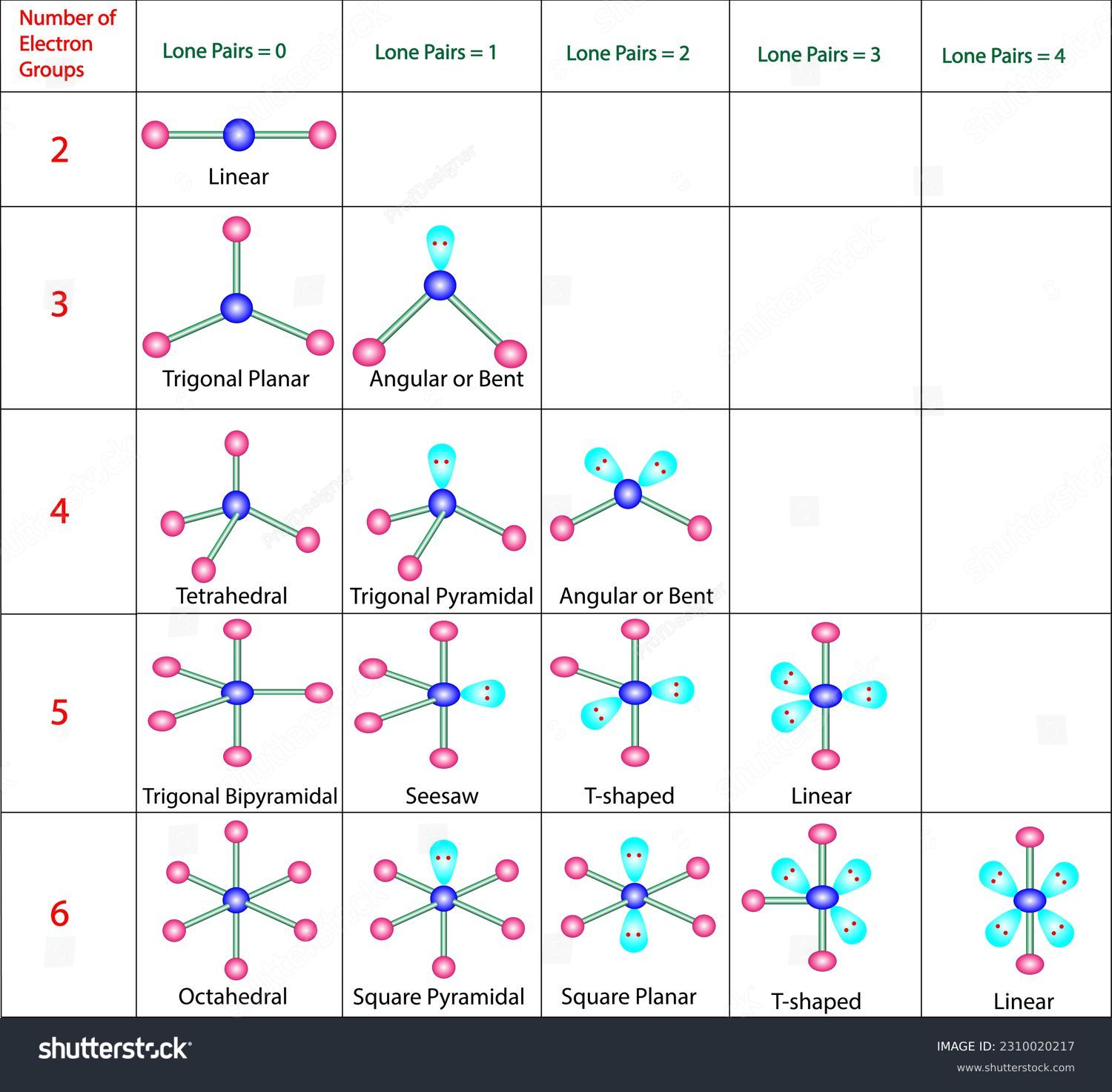
Draw the Lewis structure of the following molecules. Then, draw the VSEPR structures of the molecules. Idenyify whether the molecules are polar or non-polar. If they are polar, draw an arrow showing the direction of the dipole.
What are covalent network structures? How are the structures we covered similar to one another and different from one another?
Covalent network structures are a type of chemical bonding where atoms are bonded together by covalent bonds to form a three-dimensional network or lattice structure. In covalent network structures, each atom is bonded to its neighboring atoms by strong covalent bonds, resulting in a continuous network throughout the entire structure. These structures often have high melting points and are typically hard and rigid. Here are some common examples of covalent network structures and their similarities and differences:
1. Diamond (C):
- Structure:
Each carbon atom in diamond forms four strong covalent bonds in a tetrahedral arrangement, creating a three-dimensional network.
- Similarities:
- All carbon atoms are bonded to four other carbon atoms.
- Strong covalent bonds connect the atoms.
- High melting point due to the strength of covalent bonds.
- Hard and rigid structure.
- Differences:
- Only carbon atoms are present in the structure.
- Each carbon atom is bonded to four other carbon atoms.
2. Graphite (C):
- Structure:
Each carbon atom in graphite forms three covalent bonds in a planar hexagonal arrangement, creating layers of hexagonal rings. Weak van der Waals forces hold the layers together.
- Similarities:
- Carbon-carbon bonds are present.
- High melting point due to strong covalent bonds.
- Hard and rigid structure.
- Differences:
- Layers of hexagonal rings are present, and weak forces exist between the layers.
- Each carbon atom is bonded to three other carbon atoms within a layer.
3. Silicon Dioxide (SiO2) - Quartz and Silica:
- Structure: Each silicon atom is tetrahedrally bonded to four oxygen atoms, creating a three-dimensional network of SiO4 tetrahedra.
- Similarities:
- Silicon-oxygen bonds are present.
- High melting point due to strong covalent bonds.
- Hard and rigid structure.
- Differences:
- Silicon atoms are bonded to oxygen atoms to form a tetrahedral network.
- Different elemental composition compared to carbon-based structures.
4. Silicon Carbide (SiC) - Carborundum:
- Structure: Each silicon atom is tetrahedrally bonded to four carbon atoms, creating a three-dimensional network of SiC tetrahedra.
- Similarities:
- Silicon-carbon bonds are present.
- High melting point due to strong covalent bonds.
- Hard and rigid structure.
- Differences:
- Silicon atoms are bonded to carbon atoms in a tetrahedral network.
- Different elemental composition compared to carbon-based structures.
In summary, covalent network structures share common features such as high melting points, hardness, and rigidity, which arise from the presence of strong covalent bonds in a three-dimensional network. The differences lie in the specific atoms involved, the arrangements of these atoms, and the presence of additional forces (e.g., van der Waals forces in graphite).
What is paper chromatography? How is it able to separate components of a mixture?
Paper chromatography is a technique used for separating and analyzing mixtures of substances based on their different affinities for a stationary phase and a mobile phase. It is widely employed in chemistry and biochemistry to separate and identify components of a mixture, such as pigments in plants or dyes in ink.
The basic principle of paper chromatography involves the differential migration of components in a mixture as they interact with a stationary phase (paper) and a mobile phase (a solvent). Here's how it works:
Procedure:
1. Preparation of the Stationary Phase:
- A piece of special paper, known as chromatography paper, is used as the stationary phase. This paper is typically made of cellulose.
2. Application of Sample:
- A small spot of the mixture to be separated is applied near the base of the paper. This can be done using a capillary tube, a micro-pipette, or a similar tool.
3. Development of the Chromatogram:
- The base of the paper is immersed in a solvent or a mixture of solvents, which serves as the mobile phase. As the solvent moves up the paper by capillary action, it carries the components of the mixture with it.
4. Separation Process:
- Different components of the mixture have varying affinities for the stationary and mobile phases. The more a component is attracted to the mobile phase, the faster it will move up the paper. Conversely, components that have stronger interactions with the stationary phase will move more slowly.
5. Visualization of Bands:
- After a certain period, the paper is removed from the solvent, and the separated components are visualized. This can be done by exposing the paper to ultraviolet light, using a staining agent, or by other means depending on the nature of the components.
How It Separates Components:
- The separation is based on the principle of partition chromatography. Components with a higher affinity for the mobile phase move more rapidly through the paper, while those with a higher affinity for the stationary phase move more slowly. This differential migration results in the distinct bands or spots of separated components on the paper.
Factors Influencing Separation:
- The choice of solvent (mobile phase) and paper is crucial in achieving effective separation. The polarity of the solvent and paper can impact the separation of different compounds.
What properties do metals have in common?
1. Metallic Luster:
- Metals generally exhibit a shiny, reflective surface when freshly polished. This property is known as metallic luster.
2. Conductivity:
- Metals are excellent conductors of electricity and heat. This is due to the presence of mobile electrons in the metal structure that can move freely, carrying electrical current and thermal energy.
3. Malleability:
- Metals can be hammered or rolled into thin sheets without breaking. This property is known as malleability and is a result of the ability of metal atoms to slide past each other.
4. Ductility:
- Metals can be drawn into thin wires without breaking. This property is called ductility and is also related to the ability of metal atoms to move past one another.
5. High Melting and Boiling Points:
- Metals generally have high melting and boiling points. The strong metallic bonds between atoms require a significant amount of energy to break.
6. Solid State at Room Temperature:
- Most metals are solid at room temperature. Mercury is an exception, being a metal that is liquid at room temperature.
7. High Density:
- Metals tend to have high densities, meaning they have a relatively large mass in a given volume.
8. Electron Sea Model:
- Metals can be described using the electron sea model, where metal atoms contribute their electrons to a "sea" of electrons that move freely throughout the metal lattice.
9. Dissolving in Acid:
- Many metals react with acids to form metal salts and hydrogen gas. The reactivity of metals with acids varies.
10. Tensile Strength:
- Metals generally have high tensile strength, meaning they can withstand pulling forces without breaking.
11. Sonorousness:
- Metals produce a characteristic sound when struck, known as sonorousness. This property is often used in the context of musical instruments made of metal.
12. Oxidation State:
- Metals typically exhibit positive oxidation states in chemical reactions, where they lose electrons to form positive ions.
What is an alloy? Name all types as well as how they differ from one another.
An alloy is a mixture of two or more elements, at least one of which is a metal. Alloys are formed to enhance or modify the properties of the individual elements, resulting in a material with improved characteristics. There are different types of alloys, including interstitial alloys and substitutional alloys, each distinguished by the arrangement of atoms within the alloy.
1. Interstitial Alloys:
- Definition: In interstitial alloys, smaller atoms or ions fit into the spaces (interstices) between the larger atoms in the crystal lattice of the main metal.
- Example: Steel is a common example of an interstitial alloy. In steel, carbon atoms fit into the interstitial spaces of the iron crystal lattice.
2. Substitutional Alloys:
- Definition: In substitutional alloys, atoms of the main metal are replaced by atoms of another element of similar size. The substitutional atoms occupy the regular lattice positions.
- Example: Brass is an example of a substitutional alloy. In brass, some of the copper atoms in the lattice are replaced by zinc atoms.
3. Interstitial-Substitutional Alloys:
- Definition: Some alloys exhibit characteristics of both interstitial and substitutional alloys. In these alloys, smaller atoms occupy interstitial spaces, and larger atoms substitute for some of the main metal atoms.
- Example: Bronze is an alloy that can exhibit characteristics of both types. In bronze, tin atoms can replace some copper atoms (substitutional) while also occupying interstitial spaces.
Differences between Interstitial and Substitutional Alloys:
- Arrangement of Atoms:
- Interstitial Alloys: Smaller atoms occupy interstitial spaces between the larger atoms in the crystal lattice.
- Substitutional Alloys: Atoms of one element substitute for atoms of another element in the crystal lattice.
- Atomic Size:
- Interstitial Alloys: The atoms or ions filling interstitial spaces are typically smaller than the atoms of the main metal.
- Substitutional Alloys: The substituting atoms are generally of a similar size to the atoms they replace in the lattice.
- Effects on Properties:
- Interstitial Alloys: Tend to be harder and have altered properties due to the presence of smaller atoms in the lattice.
- Substitutional Alloys: The properties are influenced by the substituting atoms, and the alloy may exhibit a combination of properties from both elements.
Both interstitial and substitutional alloys are essential in the field of materials science and engineering, allowing the development of materials with specific desired properties, such as increased strength, corrosion resistance, or other tailored characteristics.
Given the following monomers, identify whether condensation polymerization or addition polymerization will take place. Then, draw the structure of the polymer showing two repeats.
Define each of the following: ionization energy, atomic radius, electron affinity, electronegativity. What patterns can be observed on the periodic table for these properties? and why?
1. Ionization Energy:
- Definition: Ionization energy is the energy required to remove an electron from an atom in its gaseous state. It is usually measured in kilojoules per mole (kJ/mol) or electron volts (eV).
- Periodic Trend: Generally increases from left to right across a period and decreases from top to bottom within a group on the periodic table. This is due to increasing effective nuclear charge and decreasing distance from the outer electrons to the nucleus.
2. Atomic Radius:
- Definition: Atomic radius refers to the size of an atom, usually defined as the distance between the nucleus and the outermost electron in an atom.
- Periodic Trend: Generally decreases from left to right across a period and increases from top to bottom within a group. The decrease is due to increasing effective nuclear charge, while the increase is influenced by additional electron shells.
3. Electron Affinity:
- Definition: Electron affinity is the energy change that occurs when an electron is added to a neutral atom to form a negative ion. It is an indicator of an atom's ability to accept an electron.
- Periodic Trend: Generally increases from left to right across a period and decreases from top to bottom within a group. The increase is influenced by increasing effective nuclear charge, while the decrease is due to the electron being added to a higher energy level.
4. Electronegativity:
- Definition: Electronegativity is the tendency of an atom to attract a bonding pair of electrons in a covalent bond. It is a measure of the atom's ability to attract shared electrons.
- Periodic Trend: Generally increases from left to right across a period and decreases from top to bottom within a group. Similar to ionization energy and electron affinity, electronegativity is influenced by increasing effective nuclear charge and decreasing atomic size.
Observations and Explanations for the Periodic Trends:
1. Ionization Energy:
- Left to Right (Across a Period): Increases due to increasing effective nuclear charge, making it more difficult to remove electrons.
- Top to Bottom (Within a Group): Decreases because electrons are farther from the nucleus, experiencing less effective nuclear charge.
2. Atomic Radius:
- Left to Right (Across a Period): Decreases due to increasing effective nuclear charge, pulling electrons closer to the nucleus.
- Top to Bottom (Within a Group): Increases as electrons occupy higher energy levels, and there are more electron shells.
3. Electron Affinity:
- Left to Right (Across a Period): Generally increases due to increasing effective nuclear charge, making it more favorable for an atom to gain an electron.
- Top to Bottom (Within a Group): Decreases because the added electron is farther from the nucleus, experiencing less effective nuclear charge.
4. Electronegativity:
- Left to Right (Across a Period): Increases due to increasing effective nuclear charge, resulting in a stronger pull on shared electrons.
- Top to Bottom (Within a Group): Decreases because shared electrons are farther from the nucleus, experiencing less effective nuclear charge.
How does one identify oxidation states.
Identify the oxidation state of each of the elements in the compounds shown below.
Identifying oxidation states involves assigning a numerical value (oxidation number) to each element in a compound based on a set of rules. Oxidation states represent the hypothetical charge that an atom would have in a compound if all the shared electrons were assigned to the more electronegative atom. Here are the general rules for determining oxidation states:
1. Elemental State:
- For uncombined elements, the oxidation state is zero. For example, O₂, N₂, Na, Cl₂, etc.
2. Hydrogen:
- In most compounds, hydrogen has an oxidation state of +1.
- In metal hydrides (compounds of hydrogen with metals), hydrogen has an oxidation state of -1.
3. Oxygen:
- In most compounds, oxygen has an oxidation state of -2.
- Exceptions include peroxides (e.g., H₂O₂) where oxygen has an oxidation state of -1, and compounds with fluorine, where oxygen may have positive oxidation states.
4. Alkali and Alkaline Earth Metals:
- In compounds, alkali metals (Group 1) have an oxidation state of +1, and alkaline earth metals (Group 2) have an oxidation state of +2.
5. Fluorine:
- In compounds, fluorine has an oxidation state of -1.
6. Aluminum:
- In compounds, aluminum typically has an oxidation state of +3.
7. Sum of Oxidation States:
- The sum of oxidation states in a neutral compound is zero.
- The sum of oxidation states in a polyatomic ion is equal to the charge of the ion.
8. Polyatomic Ions:
- Oxidation states of elements in polyatomic ions can be determined by considering the overall charge of the ion.
9. Transition Metals:
- The oxidation state of a transition metal may vary. In some cases, it can be determined from the overall charge of the compound or through the use of other rules.
10. Rules for Specific Compounds:
- In certain compounds or classes of compounds, specific rules may apply. For example, the sum of oxidation states in a compound must be equal to the compound's overall charge.
An octahedral metal complex absorbs light with wavelength 5.35 x 10-7 m. What color of light is absorbed? What colour would this complex appear? What is the difference in energy between the split d-orbitals?
What color will a complex appear if the energy difference between the split d-orbitals is 3.75 x 10-19 J?