Relative Formula Mass
1/7
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
8 Terms
What is the relative formula mass of an element?
The average mass of all the isotopes of the element
What is the formula for relative formula mass?
Mr = The sum of Ar of elements x number of atoms in elements
What is the formula for percentage mass of an element?
(Ar x number of atoms of that element/ Mr of the compound) x 100
What is relative Atomic Mass(Ar)?
the average mass of an atom of an element relative to one-twelfth the mass of a carbon-12 atom
How to calculate relative atomic mass(Ar)?
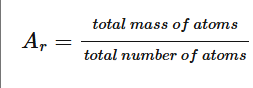
Relative Formula masses that you should know:
Substance | Formula | Mr |
|---|---|---|
Water | H₂O | 18 |
Carbon dioxide | CO₂ | 44 |
Oxygen | O₂ | 32 |
Hydrogen | H₂ | 2 |
Nitrogen | N₂ | 28 |
Methane | CH₄ | 16 |
Ammonia | NH₃ | 17 |
Hydrochloric acid | HCl | 36.5 |
Sulfuric acid | H₂SO₄ | 98 |
Sodium chloride | NaCl | 58.5 |
Calcium carbonate | CaCO₃ | 100 |
Glucose | C₆H₁₂O₆ | 180 |
Relative Atomic masses you should know:
Element | Symbol | Ar |
Hydrogen | H | 1 |
Carbon | C | 12 |
Oxygen | O | 16 |
Nitrogen | N | 14 |
Sodium | Na | 23 |
Chlorine | Cl | 35.5 |
Calcium | Ca | 40 |
Sulfur | S | 32 |
A carbon atom has a relative atomic mass of 12.
60% of the carbon atom has a mass number of 12.8, whilst the remaining 40% has a mass number
which is not known.
Calculate the mass number of the carbon isotope which is 40% of the Carbon 12 atom.
Give your answer in 2 decimal places if needed
((90 × 12) + 10z)/100
(1080 + 10z)/100
12×100 = 1210
1210 = 1080 + 10z
1210 - 1080 = 130
130 = 10z
130/10 = 13