Chemistry Quest #2
1/35
Earn XP
Description and Tags
Periodic Table Trends, Atomic Mass, Isotopes
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
36 Terms
How to calculate atomic mass
the # of protons + # of neutrons = atomic mass amu
Why decimal places?
Atomic mass of a single atom is a whole number; Element masses are based on Carbon-12 = 12 amu; Hydrogen (1 amu) is 1/12 the mass of carbon
What is an isotope
An isotope is a version of an element with a unique mass and number of neutrons
Isotopes
Nearly every element on the periodic table has at least two isotopes
Relative abundance
Isotopes have varying relative abundance: they are found in nature in differing amounts
Trace
the isotope is found in very small amounts
Calculating weighted average
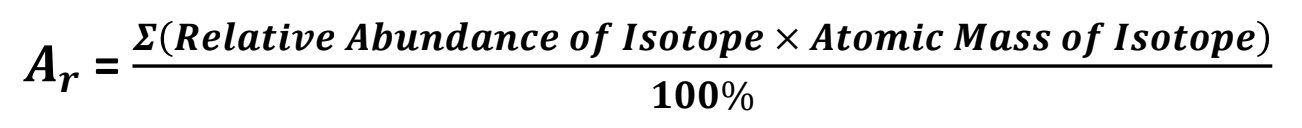
Steps of calculating weighted average
Set equation with subscripts
Sub in values
Final answer and units
Radioisotopes
A radioactive isotope
Properties of radioisotopes
Has a very unstable nucleus
Can occur naturally but many are created artificially
When the nucleus of a radioisotope decays, energy is released in the form of radiation
Atomic radius
the distance from the centre of the atom (nucleus) to the valence shell
Nuclear charge
The number of protons in the nucleus determines its positive charge.
If the number of protons changes, the strength of the nucleus’s pull on the electrons also changes.
Electron shells
Electrons only exist where electron shells or energy levels allow them to
A difference in the number of energy levels affects the distance electrons can be from the nucleus
Shielding
Electrons are attracted to protons
Inner electron shells bloc, or shield the attractive force between outer electrons and the nucleus
Electron-electron repulsion
Electrons in the same electron shell repel each other (same charge)
although, there is a greater effect due to the increased number of protons in the nucleus
TIPT - going across a period
Increased number of protons in the nucleus
Number of shells and shielding between the nucleus and valence electrons remain constant
Atomic radius decreases
TIPT - going down a group
Despite the increasing number of protons, the atomic radius increases down a group because added electron shells and greater shielding reduce the nucleus’s pull on valence electrons.
What is a trend in the periodic table
A trend shows a general pattern, but there can be exceptions, making it different than a rul
Ionic raidus
Ideally, the distance from the centre of an ion to its valence shell
It is difficult to measure consistently
Ionic radius in metals
Metals tend to lose all of their valence electrons to form cations
Ionic radius in non metals
non metals tend to gain electrons to fill their valence shell to form anions
same number of protons and electron shells, same shielding
different number of electrons resulting in more electron repulsion, and small force
Cation and anion size
A cation is smaller than its parent atom because it has fewer electron shells and less shielding, so the nucleus pulls the electrons closer.
An anion is larger than its parent atom
Cations are smaller than most anions
Ionic radius - trends across a period
Cations: Ionic radius decreases across a period.
Valence electrons are in the same energy level.
Shielding stays the same, but protons increase → stronger attraction, smaller ions.
Cations are often isoelectronic (same electron arrangement).
Anions: Ionic radius also decreases across a period.
Valence electrons in the same shell, shielding unchanged.
More protons pull electrons closer.
Anions are also isoelectronic with each other.
electron affinity
Ideally, a measure of the attractive force of an atom has for adding an electron to its valence shell
Nuclear charge - electron affinity
More protons in the nucleus = greater attraction to an additional electron
Electron shell/shielding (electron affinity)
Greater distance = decreased attraction
Greater shielding = decreased attraction
between nucleus and incoming electron
Electron affinity across a period
Increasing number of protons in the nucleus increases attraction between the nucleus and incoming electrons
number of shells and shielding between the nucleus and incoming electrons remain constant
An increasing amount of shielding between the nucleus and the incoming electron reduces the attractive force
First ionization energy
A measure of the energy required to remove ONE electron from the valence shell of an neutral atom
Nuclear charge - first ionization energy
More protons in the nucleus = stronger attraction to valence electrons.
Stronger attraction = more energy required to remove an electron.
electron shells/shielding - first ionization energy
More electron shells = valence electrons farther from nucleus.
Greater distance + more shielding = weaker attraction.
Weaker attraction = less energy needed to remove an electron.
FIE - Trends going across a period
Increases across a period.
More protons → stronger attraction to electrons.
Same shells & shielding → no added distance or blocking.
Result: harder to remove an electron.
FIE - down a group
Decreases down a group.
More shells + shielding → valence electrons farther from nucleus.
Weaker attraction → easier to remove electrons.
Despite more protons, shielding dominates.
Electronegativity
Electronegativity = an atom’s ability to attract shared electrons in a covalent bond.
It is a relative measurement (compared to other elements).
Determines bond type:
Pure covalent (equal sharing)
Polar covalent (unequal sharing)
Ionic (electron transfer)
Greater nuclear attraction to shared electrons = greater electronegativity.
Factors affecting electronegativity
nuclear charge
electron shells/shielding
Electronegativity - going across a period
increasing electronegativity
increase of protons in the nucleus
greater hold and attraction on the shared valence electrons
electron shielding/shells stay the same
E
Electronegativity - down a group
despite the increase of protons in the nucleus, electronegativity decreases since there is an increase of electron shells and shielding between the nucleus and shared valence electrons, causing less of a hold on the valence electrons