Biology - Unit 1: Molecules
1/86
Earn XP
Description and Tags
A1.1 Water, A1.2 Nucleic Acids, B1.1 Carbohydrates and Lipids, B1.2 Proteins, C1.1 Enzymes and Metabolism, C1.2 Cell Respiration, C1.3 Photosynthesis | All questions from mock tests/quizzes, revision websites and my own notes
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
87 Terms
What is the molecular structure of water?
two (+) hydrogen atoms covalently bonded to one (-) oxygen atom.
Why is water considered a polar molecule?
The electronegativity difference between oxygen and hydrogen creates a partial negative charge on oxygen and partial positive charges on hydrogens.
What allows water molecules to form hydrogen bonds?
The polarity of water molecules enables them to form hydrogen bonds with each other.
relatively weak electrostatic bonds between partially positive H of one water molecule and partially negative O of another water molecule
What is cohesion in the context of water?
Cohesion refers to water molecules sticking to each other due to hydrogen bonding, creating surface tension.
What is adhesion in relation to water?
Adhesion is the ability of water molecules to stick to other polar substances.
crucial for capillary action
What is the specific heat capacity of water?
Water has a high specific heat capacity due to the energy needed to overcome the hydrogen bonds
therefore, it can absorb and release heat with minimal temperature change
excellent temperature buffer for aquatic ecosystems
helps regulate and maintain body temperature
Why is water an excellent solvent?
Water's polarity makes it an excellent solvent for polar and ionic compounds, essential for biological processes.
What are hydrophilic substances?
Hydrophilic substances interact favorably with water, such as sugars and salts.
What are hydrophobic substances?
Hydrophobic substances do not interact well with water, including fats and oils.
How do the boiling and melting points of water compare to methane?
Water has a boiling point of 100°C and a melting point of 0°C, while methane has a boiling point of -161.5°C and a melting point of -182.5°C.
What makes water an effective coolant?
Water's high specific heat capacity and high latent heat of vaporization make it an excellent coolant.
How does water transport glucose and amino acids in blood?
Glucose and amino acids are dissolved directly in the water component of blood plasma due to the polarity of water.
How are cholesterol and fats transported in blood?
Cholesterol and fats are transported in lipoproteins due to their hydrophobic nature.
What role does water play in thermoregulation for humans?
Sweating is a crucial mechanism for thermoregulation, utilizing water's cooling properties.
What factors can be investigated in experiments on water's cooling properties?
Factors include surface area of water exposed, air movement, initial water temperature, and humidity.
What is the significance of water resources in relation to population growth?
Equitable distribution of water resources is critical for drinking, agriculture, industry, and ecosystem maintenance.
What are the two main types of nucleic acids?
DNA (deoxyribonucleic acid) and RNA (ribonucleic acid).
What are the three components of a nucleotide in DNA/RNA?
A phosphate group (acidic and negatively charged), a pentose sugar (made up of 5 carbon atoms), and one of four nitrogenous base.
Describe the structure of DNA
Deoxyribose as pentose sugar
Double-helix
Two twisted, anti-parallel strands
Covalent bonds form a sugar-phosphate backbone
Hydrogen bond between bases (A&T have 2, C&G have 3)
Describe the structure of RNA
Ribose sugar
Single stranded
Uracil instead of thymine
Sugar-phosphate backbone
Describe DNA replication
Helicase unwinds the DNA double helix and breaks hydrogen bonds between bases
Each single strand acts as a template for a new strand, it is a semi-conservative process
DNA polymerase adds nucleotides to the template strand, added through complimentary base pairing
Two semi-conservative copies of the template DNA are created
Why is DNA a good way to store genetic information?
complimentary base pairing allows for mostly accurate replication
takes up little space and material
can be any length, large range of possible genetic information
information stored in coded for that is universal (universal genetic code)
Outline the conservation of genetic code
There are 64 different codons, most specify for a specific amino acid
All organisms share the same genetic code with few exceptions
The same codons code for the same amino acids in all organisms
May trace back to the last universal common ancestor (LUCA)
What is polymerase chain reaction (PCR)?
Method of amplifying (making many copies of) a DNA sequence from a small sample.
is a repeated cycle, repeating it can make multiple copies of a single sample
Describe the process of PCR
Denaturing: DNA is heated to 95C, breaking the hydrogen bonds and separating DNA strands
Annealing: temp reduced to53C, allowing primers to bind to both DNA strands while exposed
Extending: Sample is heated to 75C allowing Taq DNA polymerase to attach to primer and add free nucleotides
Two semi-conservative, double stranded copied of template DNA sample are produced
What is Taq DNA polymerase?
A special type of heat-stable DNA polymerase obtained from bacterium found near hot springs, it can function in high temperatures which speeds up the replication process
What are primers?
Short DNA strands with the base sequence needed that bind at the point where DNA polymerase should start copying.
one primer for each strand
Outline the purpose of the enzymes involved in DNA replication
Helicase: unwinds the double helix and unzips/separates strands by breaking hydrogen bonds
DNA polymerase: makes a complimentary new strand by adding free DNA nucleotides to template strand through complimentary base pairing
What is gel electrophoresis?
Technique used to separate fragments of DNA according to size, producing DNA profiles.
Outline the process of gel electrophoresis
DNA fragments are placed in wells in a porous gel
Electrodes at attached to tank (DNA is negatively charged) DNA fragments move towards positively charged electrode
DNA fragments are separated by size (smaller fragments move faster and further through gel than larger ones)
Pattern of the bands created in the gel by DNA can be used to create DNA profiles, which can be compared
What is DNA profiling?
Comparing combinations of DNA sequences unique to each individual.
DNA sample is taken → primers chosen to promote amplification of short tandem repeats (STRs) during PCR → pattern of bands create DNA profile usually unique to each individual
How is DNA profiling used?
Forensic investigations: DNA profiles can provide evidence by comparing suspect’s DNA to DNA samples found at crime scenes
Paternity tests: DNA profiles used to determine parents of a child. DNA profile of mother, child, and suspected father are compared. Child shares DNA of mother and father. The bands that do not match the mother must therefore match the father.
What is transcription and the process?
Synthesis of mRNA using DNA as a template.
RNA polymerase pries apart template DNA, bines to a site at the start of a gene and transcribes it
Using complimentary base pairing, sequence of coding strand is copied into mRNA (adenine is paired with uracil)
Paired until end of gene, where mRNA is released
Explain the purpose of transcription for the expression of genes
gene is expressed when the information it holds is used to generate an observable characteristic within organism/cell
cells are specialized for particular functions, only develop characteristics it needs
What is translation?
Synthesis of a polypeptide using mRNA to determine amino acid sequence (takes place in ribosomes)
Outline the process of translation
mRNA binds to a site on small ribosomal subunit. Each codon codes for 1 amino acid
tRNA molecules are present around ribosome in large numbers. Each tRNA carries an anticodon with corresponding amino acid
tRNA molecules have 3 binding sites on large subunit, only 2 bind at a time. tRNA can only bond if it has the complimentary anticodon to mRNA. They link together by forming hydrogen bonds
first anticodon is start tRNA carrying methane
Amino acids carried by tRNA bonded by peptide bonds (elongation) and repeat until stop codon is reached and polypeptide is formed
What are monosaccharides?
Monosaccharides are single sugars; e.g glucose and fructose;
Disaccharides consist of two monosaccharides linked together; e.g. lactose and sucrose;
Polysaccharides consist of many monosaccharides linked together; e.g. starch and glycogen;
Form - can form 5 carbon rings, pentose sugars, like deoxyribose;
can form 6 carbon rings, hexose sugars, like glucose;
Function - Glucose
Glucose is very soluble so can be dissolved in water e.g. in blood, and transported;
it is chemically stable and can be stored as a polysaccharide as starch or glycogen, so it does not effect osmosis;
it has a high energy yield when broken down to release energy in respiration;
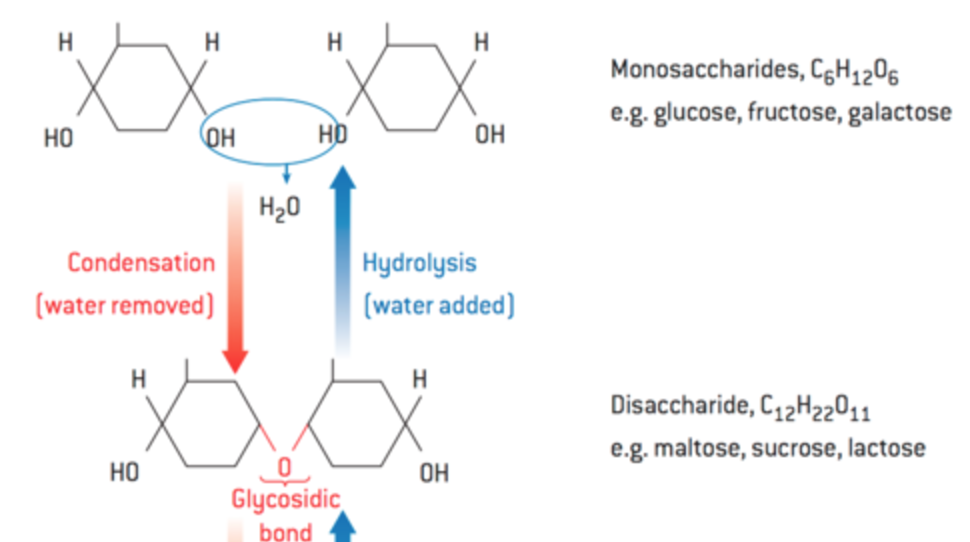
What is a disaccharide?
A carbohydrate formed by the combination of two monosaccharides.
What are polysaccharides?
Complex carbohydrates formed by many monosaccharides linked together.
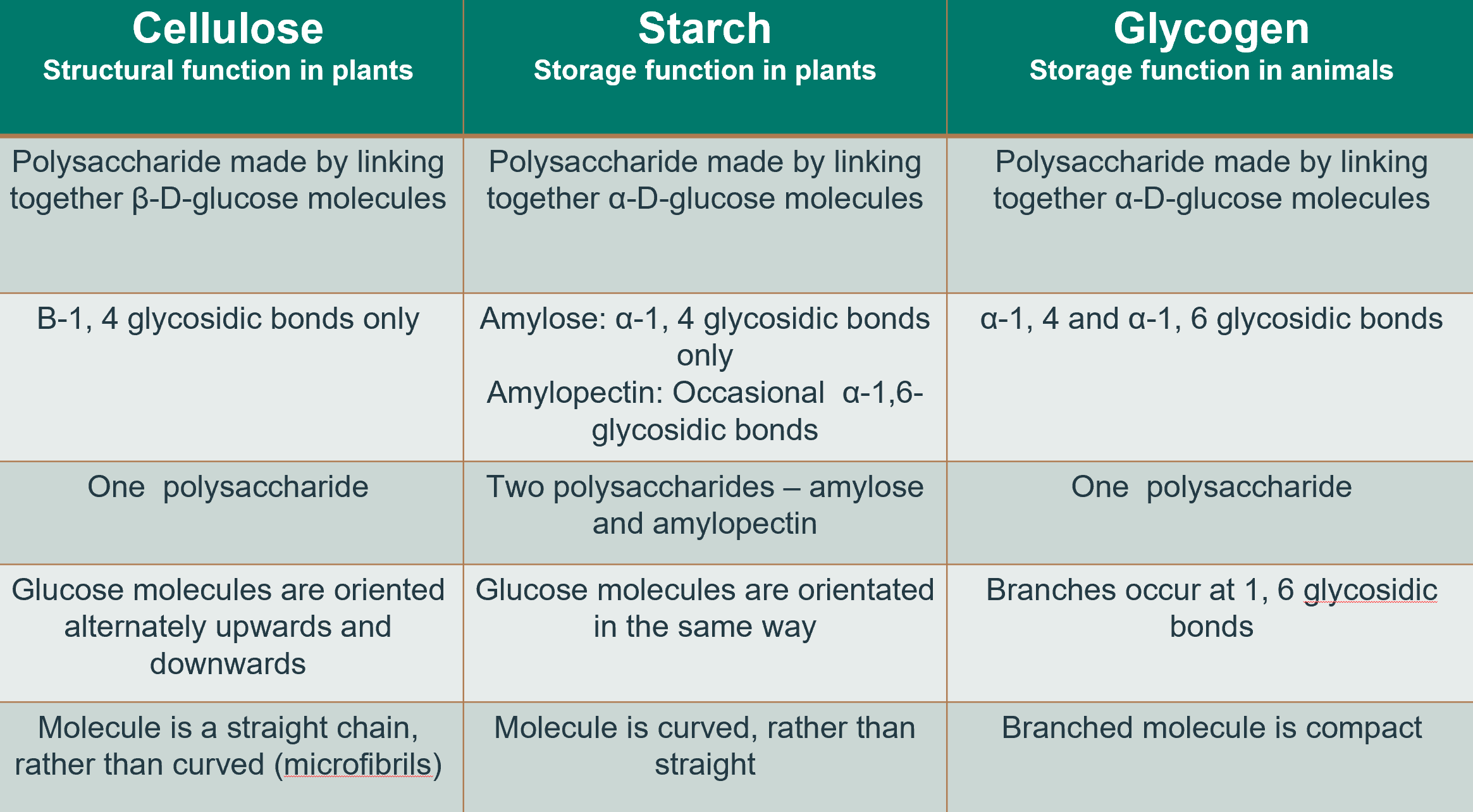
What is cellulose?
A structural polysaccharide in plant cell walls, made of glucose units connected by β-1,4 glycosidic bonds.
What are the two types of glucose polymers in starch
Amylose (linear) and amylopectin (branched).
What is the primary function of glycogen?
It serves as the primary carbohydrate storage molecule in animals.
What distinguishes saturated fatty acids from unsaturated fatty acids?
Saturated fats; have only single bonds between carbons and no double bonds;
monounsaturated have one double bond;
polyunsaturated have 2 or more double bonds;
double bonds lower melting point;
polyunsaturated fats are oils at rooms temp;
fatty acids are used as storage of energy in plants; and insulation and storage in endotherms (animals that maintain body temp;
What are cis and trans isomers?
Cis isomers have hydrogen atoms on the same side of a double bond; trans isomers have them on opposite sides.
What are triglycerides?
The most common form of stored fat, formed from three fatty acids and one glycerol molecule.
formed by 3 condensation reactions between glycerol and 3 fatty acids
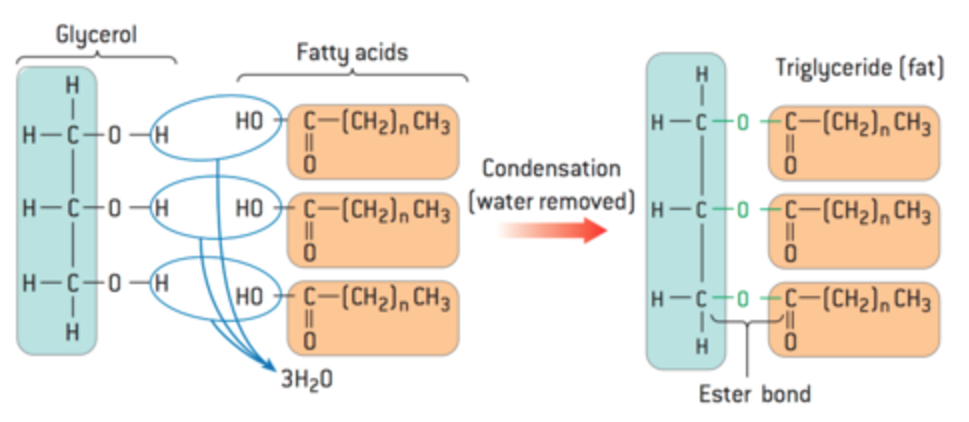
Why are lipids more suitable for long-term energy storage than carbohydrates?
They have higher energy density, are hydrophobic, and help regulate metabolism.
What are monomers? What are polymers?
Monomers are single units; e.g. monosaccharides or amino acids;
Polymers are long chains of repeating monomers; e.g. polysaccharides like starch;
Name the polymer and macromolecule of these monomers: glucose, amino acid, nucleotides, fatty acids & glycerol
monomer → polymer → macromolecule
glucose → polysaccharide → amylose & amylopectin
amino acid → polypeptide → proteins
nucleotide → polynucleotide → DNA/RNA
fatty acids & glycerol → triglyceride → fats & oils
what is the difference between condensation and hydrolysis?
condensation: two molecules combine, releasing a water molecule
hydrolysis: water added to break down a large molecule
How is cellulose used in plants? What is its structure and function?
cellulose is a polysaccharide made by linking beta glucose together from carbon 1-4;
causing a straight chain;
glucose in bundles and cross-linked with hydrogen bonds;
leads to high tensile strength for cell walls;
to prevent bursting of cells;
What is carbon?
The fundamental element forming the basis of all life, known for its ability to create multiple bonds, allowing diverse organic molecule formation.
What are glycoproteins? How are glycoproteins used in cell-cell recognition?
Glycoproteins are proteins with short chains of sugars at the end; e.g. a polysaccharide end;
they are used to help recognise cells;
as receptors;
they stick out of cells; e.g. ABO antigens which are blood glycoproteins;
they allow for cells to recognise self or non-self cells;
What are lipids? What are their properties?
A diverse range of organic compunds with chains of hydrocarbons;
not soluble (only sparingly) in aqueous solvents;
lipids include fats, oils, waxes and steroids;
they are hydrophobic;
What is alpha vs beta glucose?
Alpha: OH is symmetrical, on the bottom; used in glycogen and starch
Beta: OH is asymmetrical, rightmost OH is up; found in cellulose
How are the properties of triglycerides useful in long-term storage and insulation
Triglycerides contain a lot of energy per gram;
are also good insulators;
makes them useful as long-term energy stores and also as thermal insulators;
for organisms such as seals; which need to maintain body temp in cold conditions;
What are phospholipids? How are lipid bilayers (in cell membranes) the consequence of the properties of phospholipids?
phospholipids are part lipid and part phosphate;
the lipid region is hydrophobic and the phosphate group is hydrophilic;
this means they are amphipathic as they have both polar and non-polar properties;
they arrange in a bilayer forming the cell membranes of living things;
What molecules can pass directly through the phospholipid bilayer?
non-polar substances;
as the cell membrane is largely non-polar;
oestradiol and testosterone are non-polar, steroid hormones and can pass directly through the membrane;
steroids have a ring shape;
Describe the three main polysaccharides (name, function, formation, structure, # of polysaccharides, orientation)
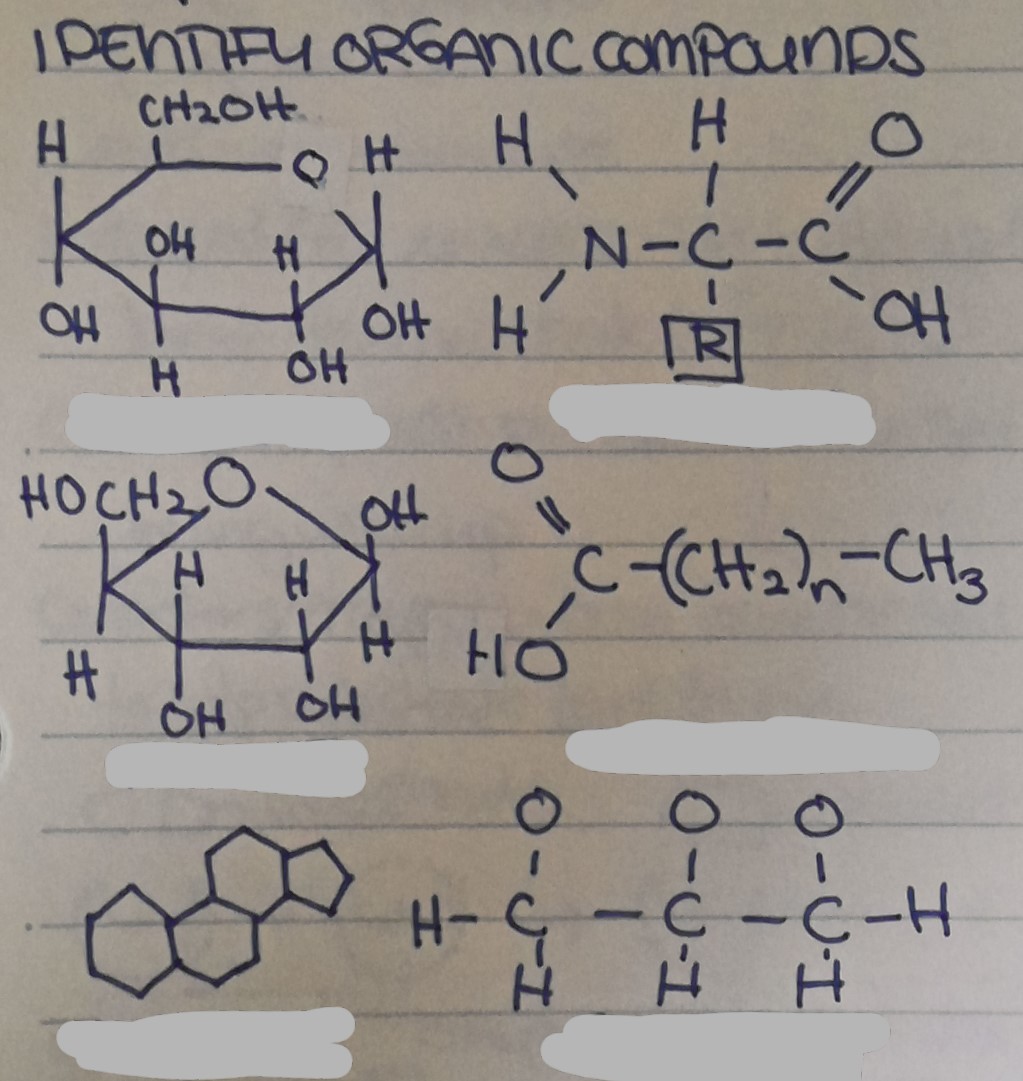
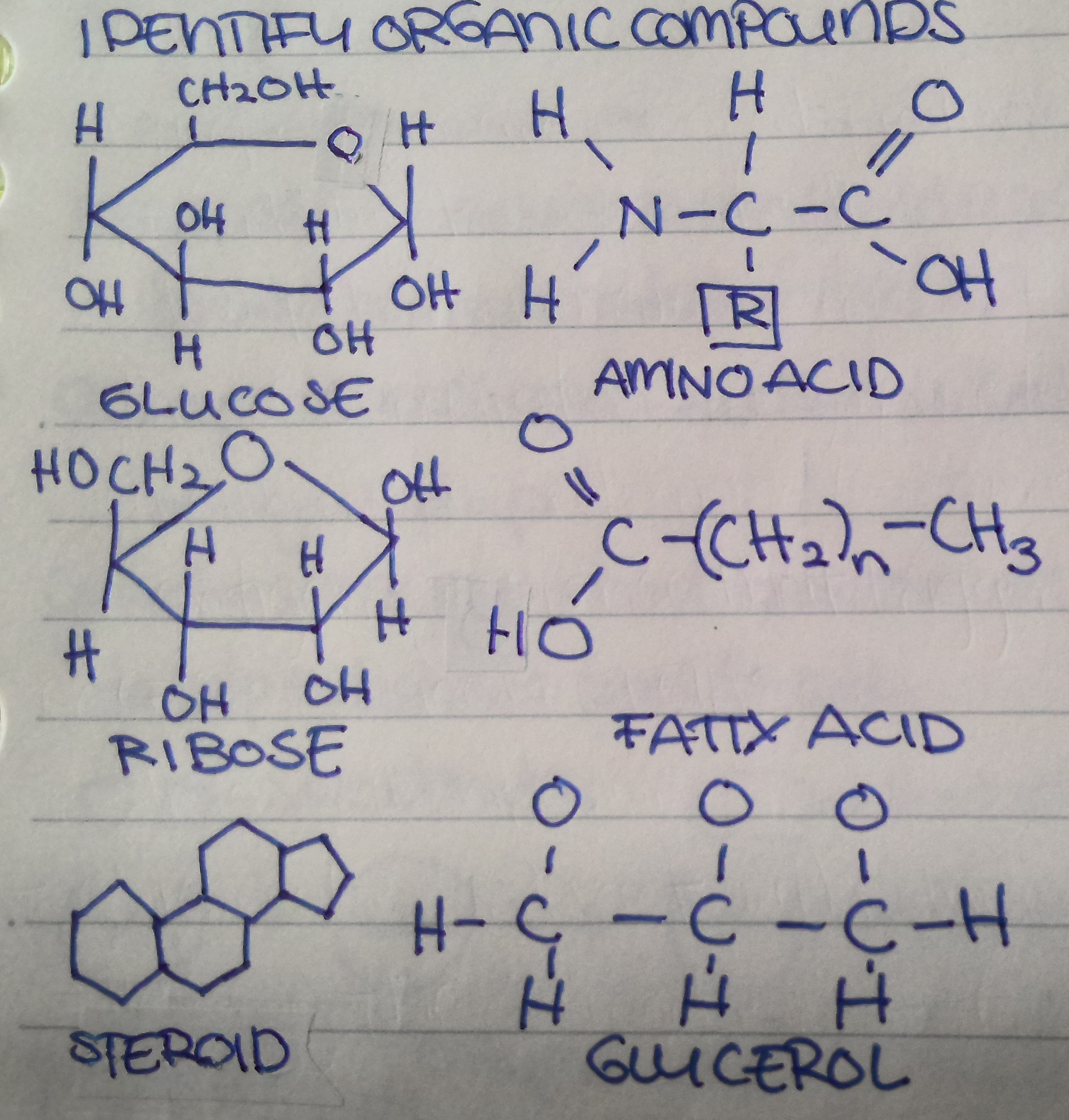
Name the organic compound from top to bottom, left to right.
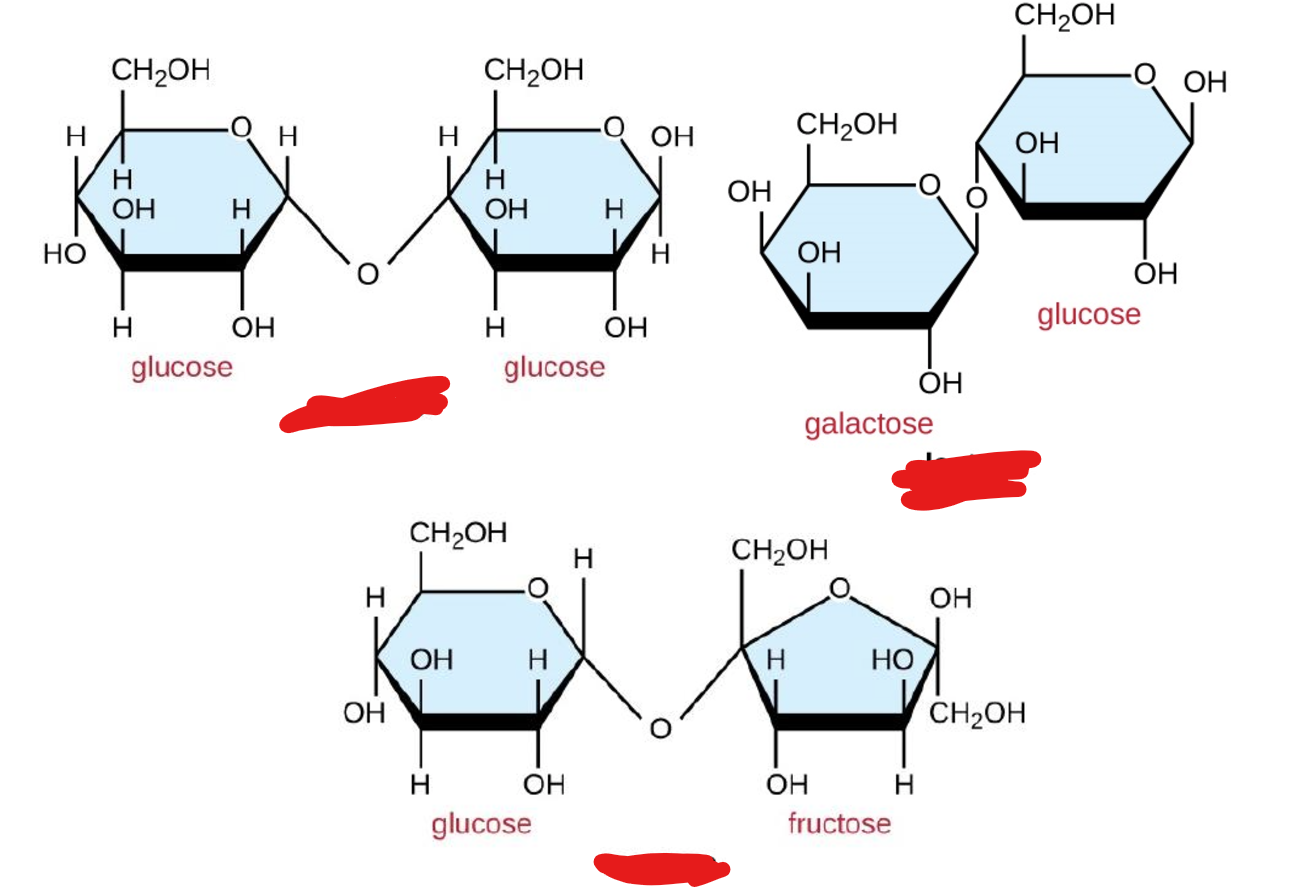
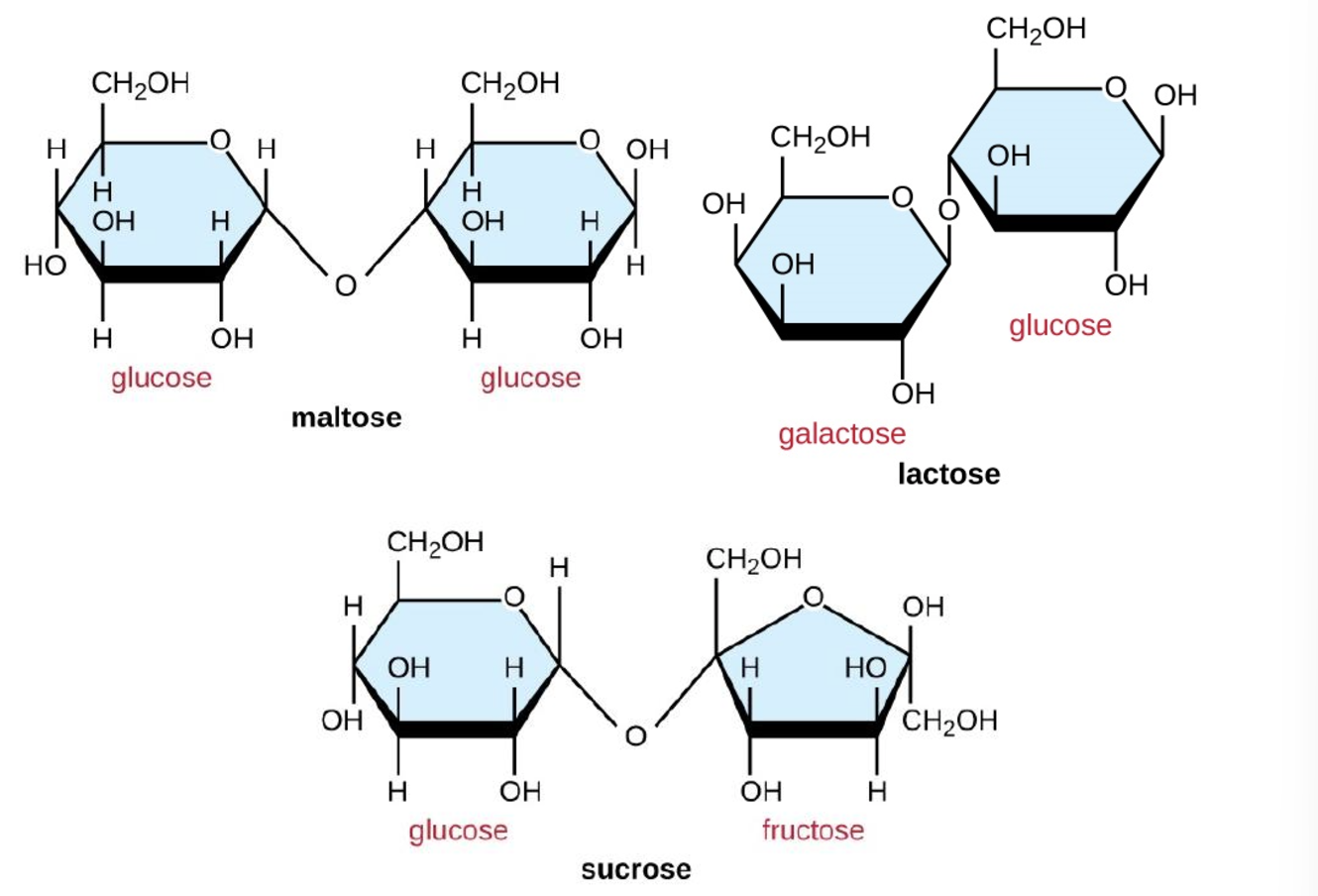
Name each disaccharide
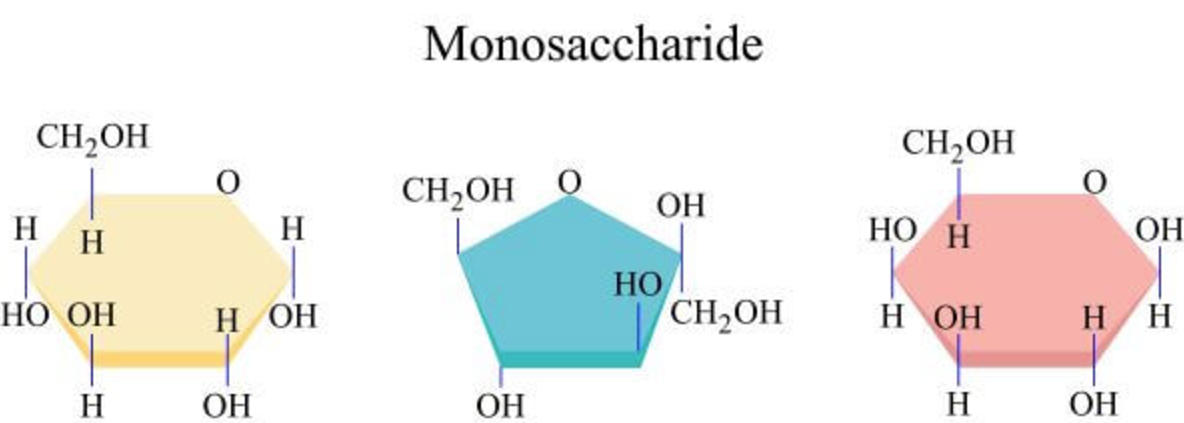
Name each monosaccharide
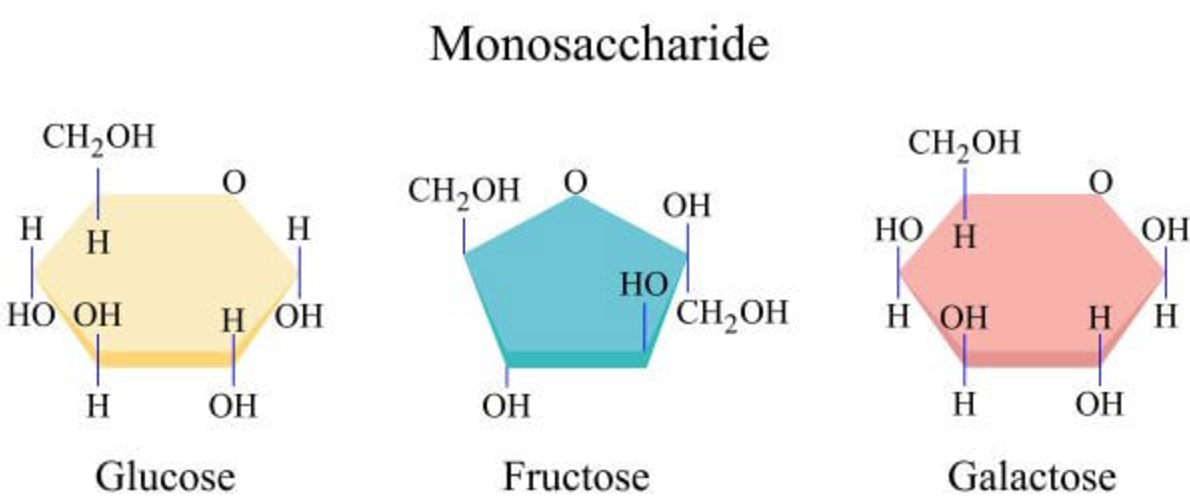
Give 3 examples of monosaccharides, disaccharides, and polysaccharides
Mono: Glucose, galactose, fructose
Di: Lactose, maltose, sucrose
Poly: Cellulose, glycogen, starch
Compare lipid and sugar energy use
Similarities:
both contain a lot of chemical energy
polysaccharides and lipids both insoluble
both burn cleaner than proteins (no nitrogenous waste
Differences:
Lipids contain more energy per gram
Lipids are therefore lighter than sugars of equal yield
Carbohydrates more easily digested (short-term)
Lipids less readily digested (long-term storage)
Mono/disaccharides are water soluble, lipids are not
Sugars easier to transport in bloodstream to sites of use
State the types of bonding for sugars, proteins, and lipids
sugars: glycosidic linkage
proteins: peptide bonds
lipids: ester linkages
State the functions of lipids
Storage of energy (triglycerides)
Hormonal signallling (steroids)
Insulation (triglycerides; sphingolipids)
Protection of internal organs
Structure of membranes (phospholipids)
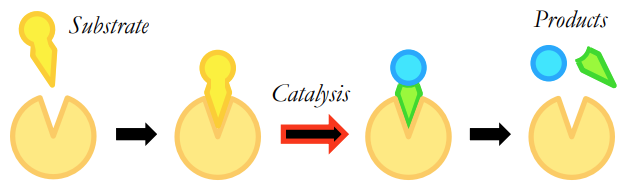
What is the lock and key model?
• Enzyme and substrate complement each other precisely in terms of both their shape and chemical properties
• The active site and the substrate will share specificity
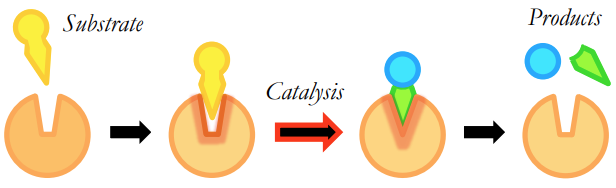
What is the induced fit model?
• Active site is not a rigid fit for the substrate and changes its conformation to better accommodate the substrate
• This stresses the substrate bonds and induces catalysis
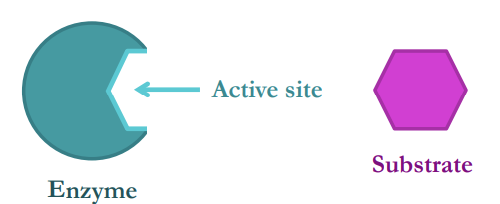
What are enzymes and how are they involved in catalysis?
An enzyme is a globular protein which accelerate chemical reactions in living organisms by lowering the activation energy (i.e. it is a biological catalyst)
• Enzymes are not consumed by the reactions and can be re-used
The molecule(s) the enzyme reacts with is called the substrate, which binds to a complementary region on the enzyme’s surface (active site)
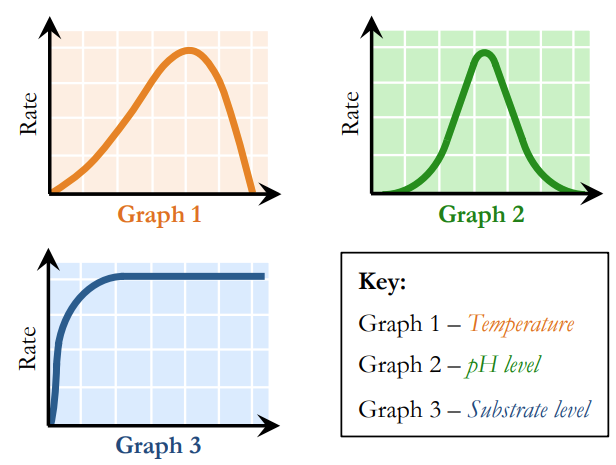
What factors affect enzyme activity and how?
Temperature
Increases enzyme activity (more kinetic energy = more collisions)
Enzyme activity peaks at an optimal temperature
Higher temperatures decrease activity (causes denaturation)
pH
Enzyme activity is highest at an optimal pH range
Activity decreases outside of this range (due to denaturation) Substrate
Concentration
Increases enzyme activity (more particles = more collisions)
At a certain point, activity plateaus (saturation of active sites)
What are immobilized enzymes and how are they used industrially?
Immobilised enzymes are often used in industrial practices
They are fixed to a static surface to prevent enzyme loss
This improves separation of product and purity of yield
One application for immobilized enzymes is the production of lactose-free milk and associated dairy products
Lactase (enzyme) digests lactose into glucose / galactose
Lactase is fixed to an inert surface (e.g. alginate beads)
Milk is passed over this surface to become lactose free
There are several benefits associated with lactose-free milk:
Provides a source of dairy for lactose-intolerant people
Increases sweetness of milk (less need for sweeteners)
Reduces crystallization and production times for cheese
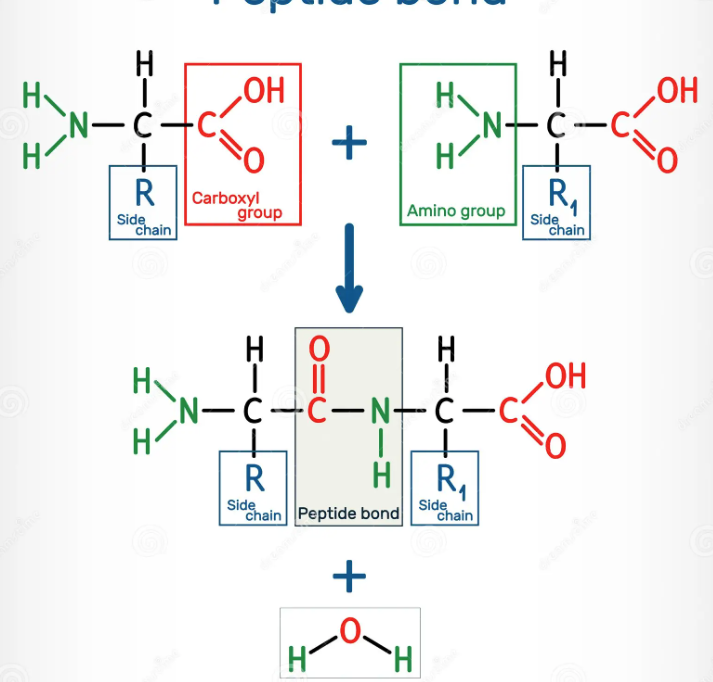
What are the three groups of a generalized amino acid and How are dipeptides formed?
Amine group, side chain, carboxyl group
Condensation reaction links together two amino acids
When this occurs with 3 or more amino acids, it forms a polypeptide
What are the differences between essential, conditionally essential and non-essential amino acids?
non essential:
can be produced by the body via other amino acids or breakdown of proteins
not requires in diet, but still have important functions
conditionally essential:
usually produced by the body, but may need to be obtained from food due to sickness/other factor
essential:
cannot be produced by the body
obtained from nutrition (proteins broken down via digestion)
necessary for growth, maintenance of body tissues and organs
What is protein denaturation and what factors cause it/how?
Denaturation is the process by which proteins lose their 3D conformational structure, often leading to loss of function.
Caused by heat:
vibrations occur within protein that break intermolecular bonds and cause conformation to change. Almost always irreversible. (Eg. heating egg white causes albumin to become denatured and insoluable)
pH:
occurs when pH deviates too far from the optimum, changes charge of R-group and breaks ionic bonds and forms new ones.
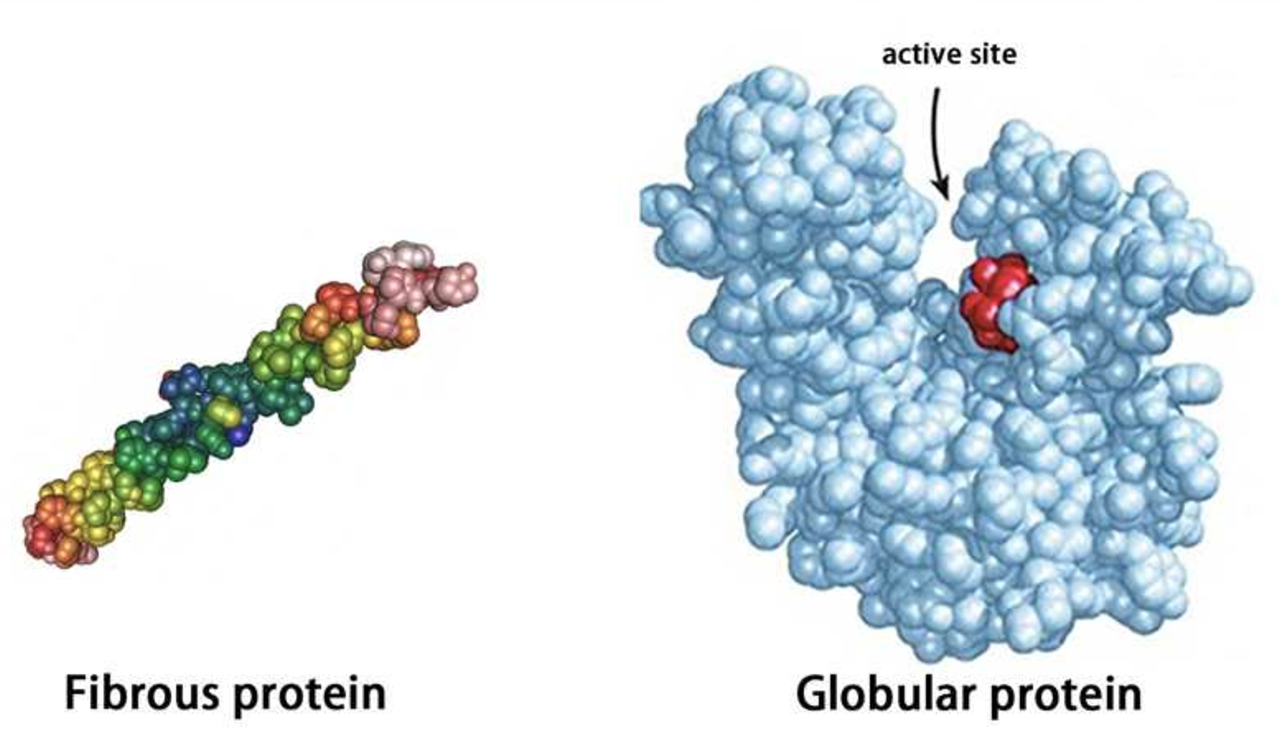
What are the differences between globular and fibrous proteins?
Globular protein:
have folded polypeptides (resulting in rounded shape)
soluble in water
Quaternary Structure: Some globular proteins consist of multiple polypeptide chains or subunits, held together by various interactions.
dynamic structures, globular proteins are highly versatile and play various roles in biological processes.
(Enzymes, Transport Proteins, Regulatory Proteins, Storage Proteins)
Fibrous:
elongated, thread-like structures
generally insoluble in water
mainly play roles in maintaining the structural integrity of cells and organisms
can be wound together to withstand forces and stretch/recoil under tension
(Collagen, Keratin, Elastin)
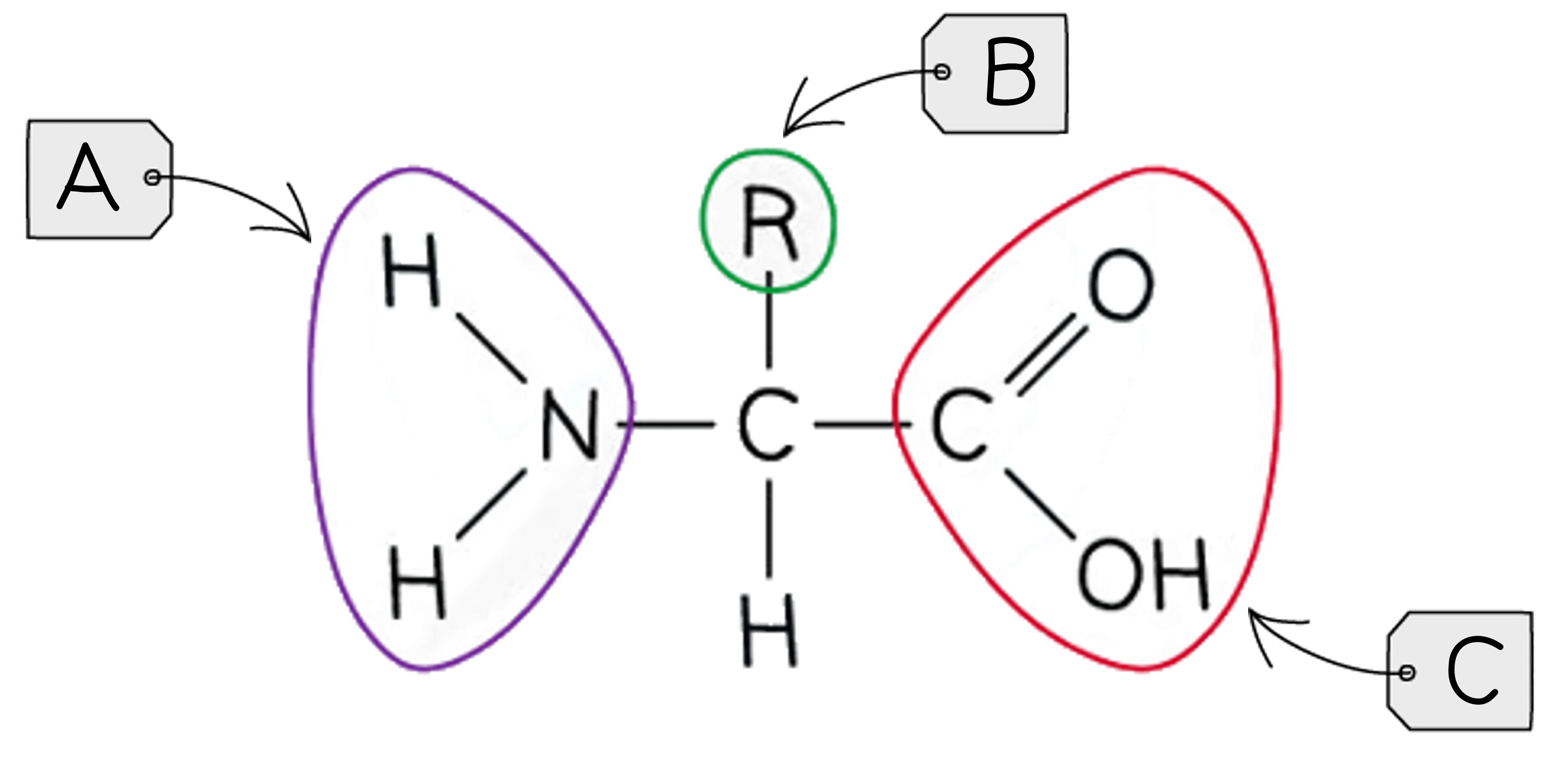
Describe the general structure of an amino acid.
An amino acid consists of a central carbon (alpha carbon) bonded to an amine group (-NH2), a carboxyl group (-COOH), a hydrogen atom, and an R group (which varies among amino acids).
Why is a well-balanced diet important for protein synthesis?
Nine essential amino acids must be obtained from our diets. While meat contains all nine, vegetarian or vegan diets require variety to ensure regular consumption of essential amino acids.
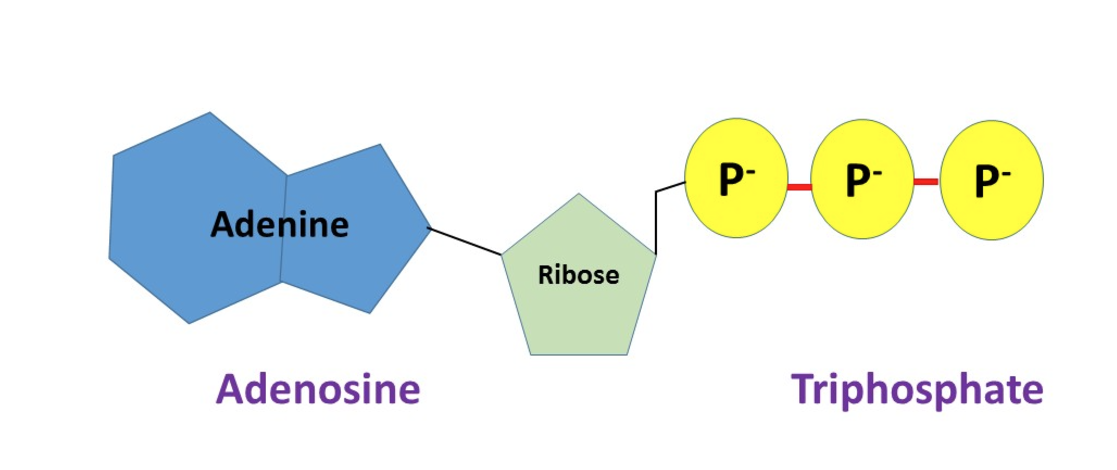
What is Adenosine Triphosphate (ATP)?
Adenosine Triphosphate (ATP) is a small and soluble molecule that provides a short-term store of chemical energy that cells can use to do work.
- phosphorylated nucleotide.
- Universal energy currency: it is used in all organisms and can be reused countless times for different reactions.
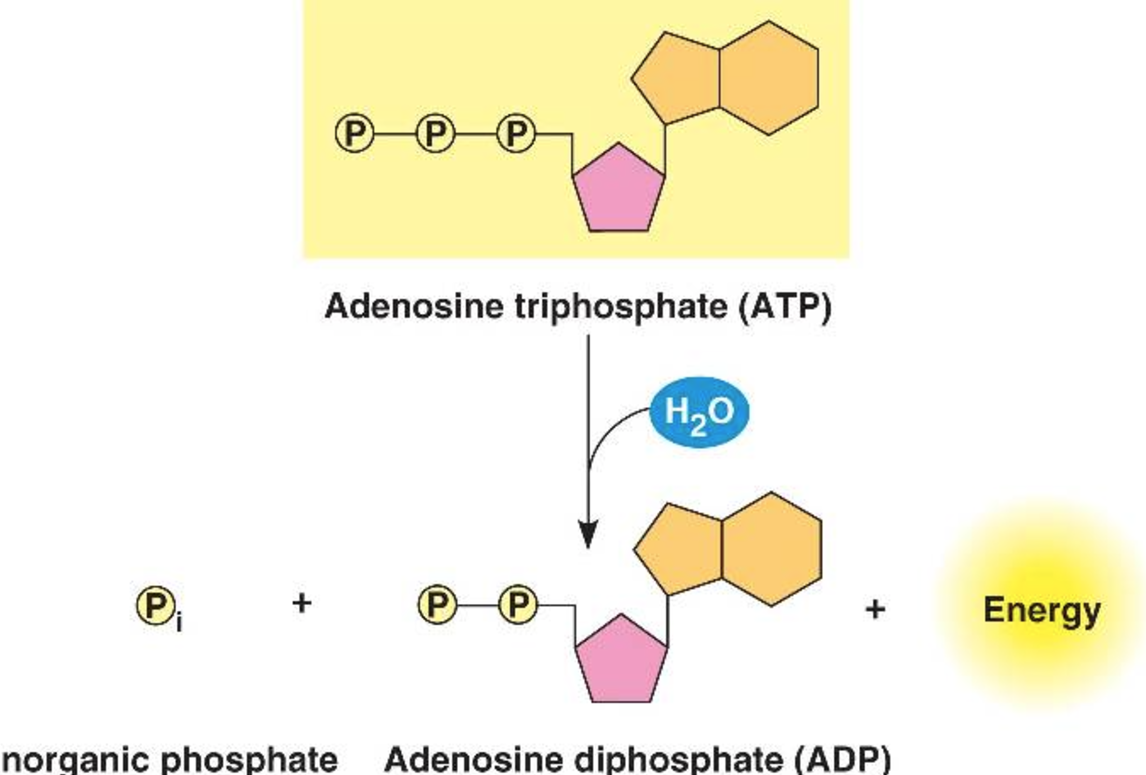
What happens when ATP is hydrolysed/condensed (synthesis)?
When ATP is hydrolysed, ADP and a phosphate ion (Pi) are produced, and energy is released.
Water is released when ATP is formed because ATP synthesis is a condensation reaction.
What is the purpose of cell respiration?
Cell respiration is a catabolic process.
The purpose of cell respiration is to release energy in usable forms from the chemical energy stored in food, such as glucose.
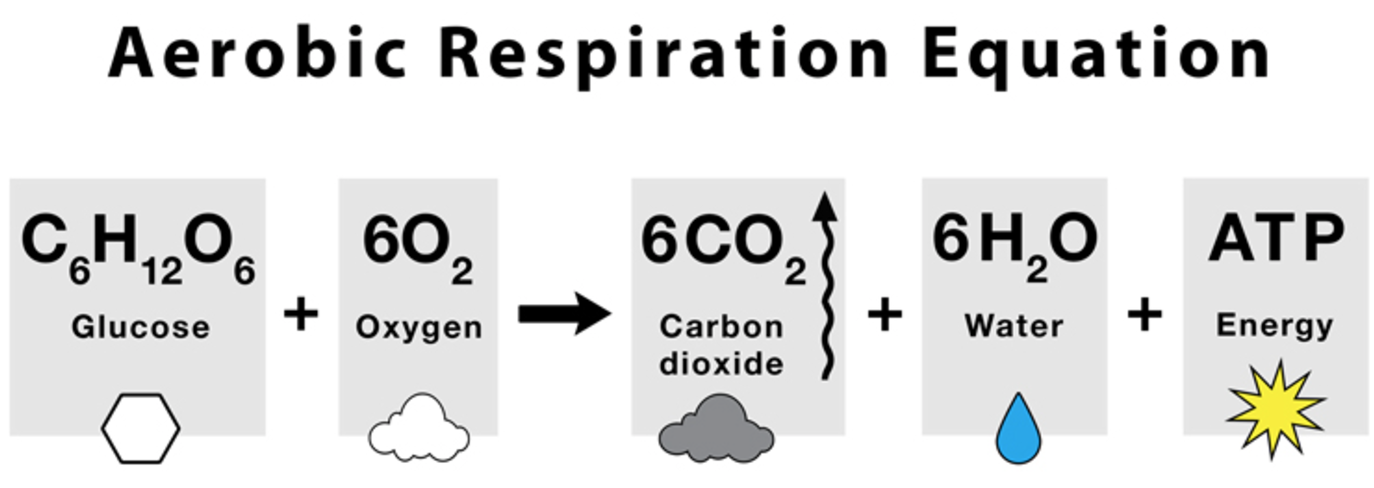
Define the term aerobic respiration.
Aerobic respiration is the process of breaking down (hydrolysis) a respiratory substrate, such as glucose, in the presence of oxygen to a large amount of ATP, water, and carbon dioxide.
- occurs in the mitochondria
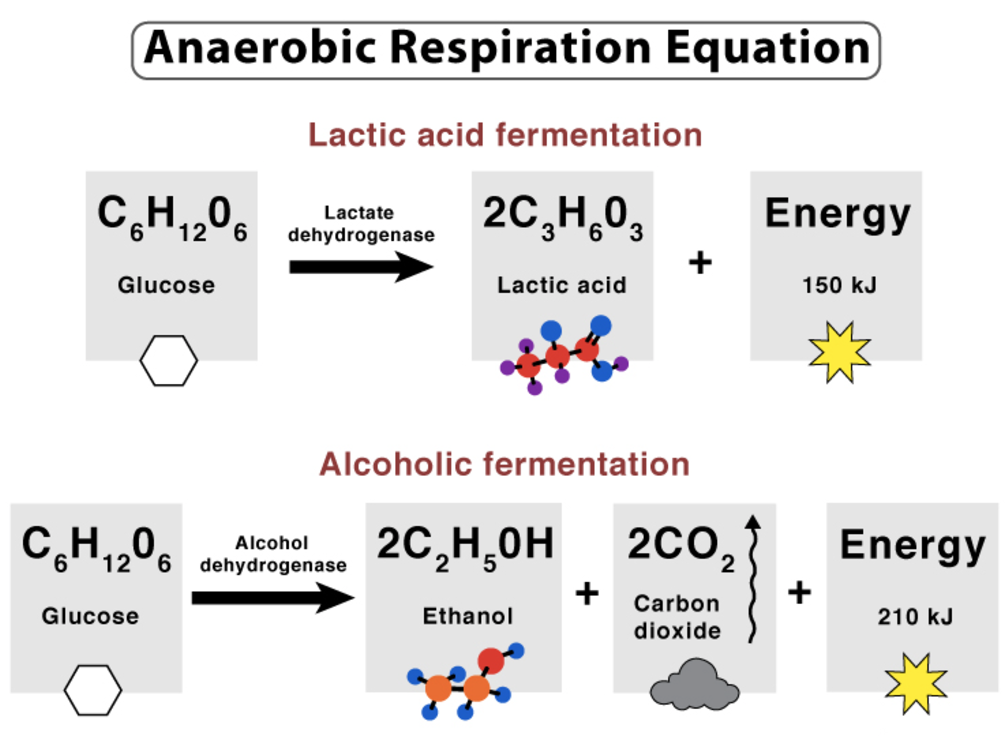
Define anerobic respiration.
The process of breaking down a respiratory substrate (glucose) in the absence of oxygen to produce:
Humans: lactate + carbon dioxide + 2 ATP
Yeast: ethanol + carbon dioxide + 2 ATP
What are gene mutations and how do they occur?
Structural changes/ alterations in the DNA sequence of an organism that can occur randomly in a genome.
May be caused by
errors during DNA replication and repair
exposure to mutagens (UV/ionizing radiation, virus)
random events
What types of mutations are there (in terms of effects)?
neutral mutation: mutation in a non-coding region of the gene
silent mutation: mutation with no effect on protein synthesized due to degeneracy of genetic code.
missense mutation: when the code is mutated to produce a different protein, which may result in new traits
nonsense mutation: when the code is mutated to produce a stop codon, which may result in a biological process being hindered
What is sickle cell disease and how is it caused? What are the benefits and downsides?
The amino acid change (Glu → Val) alters the structure of haemoglobin, causing it to form insoluble fibrous strands
The insoluble haemoglobin cannot carry oxygen as effectively, causing the individual to feel constantly tired
The formation of fibrous haemoglobin strands changes the shape of the red blood cell to a sickle shape
The sickle cells may form clots within the capillaries, blocking blood supply to vital organs and causing myriad health issues
The sickle cells are also destroyed more rapidly than normal cells, leading to a low red blood cell count (anaemia)
Those with sickle cell a
What are types of mutations (in terms of codons)
substitution:
addition/subtraction:
frameshift:
Explain the causes of gene mutations and distinguish between mutations that occur in germ cells vs. somatic cells. Include the implications for offspring.
Gene mutations can be caused by various factors including:
Environmental influences such as radiation, chemicals, and viruses
Errors during DNA replication
Distinguish between mutations in germ cells vs. somatic cells:
Mutations in germ cells (sperm and egg cells)
Can be passed on to offspring
May result in inherited genetic disorders
Mutations in somatic cells (body cells)
Not inherited by offspring
Can lead to diseases such as cancer in the individual
Germ cell mutations are significant as they can affect the genetic makeup of future generations.