Immunology - Lecture 17 - Function of Secondary Lymphoid Organs
1/21
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
22 Terms
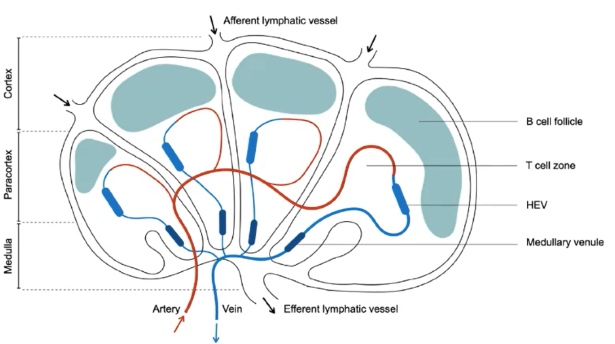
How do T cells and B cells enter secondary lymphoid organs, and what are their distinct zones within a lymph node?
B Cells outnumber T Cells for any given antigen:
B cells (100-1000) recognize antigens directly.
T cells (5-10) require antigen processing.
Zones:
B cells reside in outer B cell follicles.
T cells are in inner T cell zones.
Zones are adjacent but physically separated.
Entry:
Lymphocytes (T and B cells) enter via high endothelial venules (HEV).
HEVs express molecules to facilitate lymphocyte entry.
Lymphatic Vessels:
Afferent lymphatic vessels carry fluid, antigens, dendritic cells, and innate immune cells (not lymphocytes).
Efferent lymphatic vessels drain adjacent tissues.
This organization supports efficient immune cell interactions with antigens and each other.
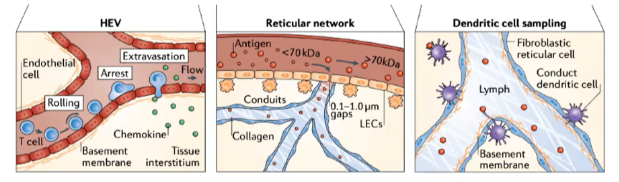
What makes HEVs unique in allowing lymphocyte entry into lymph nodes?
HEVs express addressins such as GlyCAM-1 and PNAd.
These addressins interact with L-selectin on lymphocytes.
Creates a sticky interaction with the endothelial wall, facilitating rolling and migration into lymph nodes.
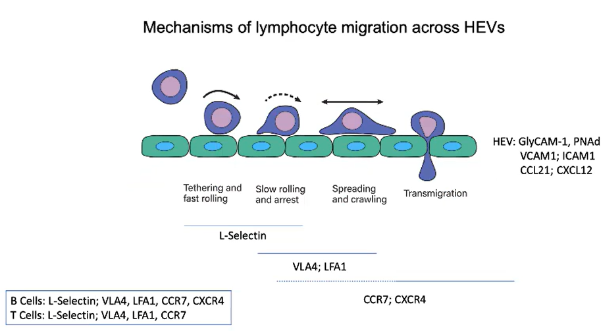
What initiates the tethering and fast rolling of lymphocytes on HEV endothelium?
L-selectin on B and T cells binds to GlyCAM-1 and PNAd on HEVs.
This binding enables lymphocytes to tether and roll along the endothelial wall.
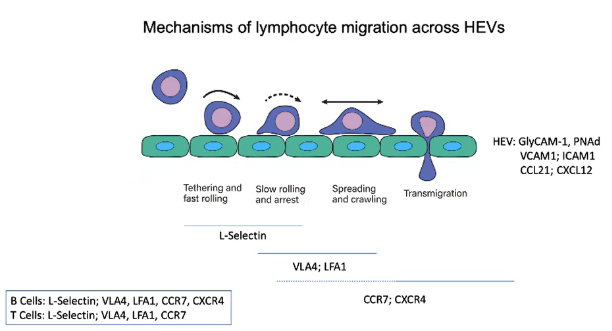
How do chemokines on HEVs influence lymphocyte migration?
CCL21 binds to the CCR7 receptor on lymphocytes.
CXCL12 binds to the CXCR4 receptor on lymphocytes.
These interactions activate integrins, enhancing adhesion and stopping lymphocyte rolling.
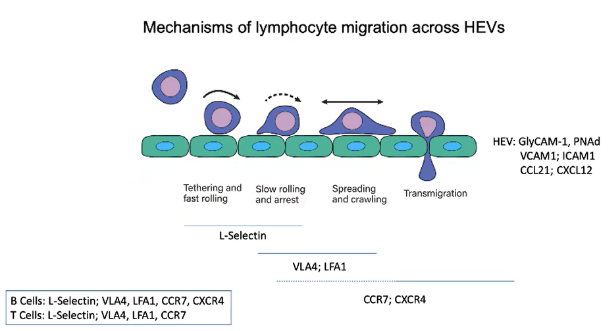
Which integrins are activated on lymphocytes to stop rolling and promote HEV entry?
VLA4 and LFA1 integrins are activated on lymphocytes.
VLA4 binds to VCAM1 and LFA1 binds to ICAM1 on HEVs.
This binding creates strong adhesion, stopping rolling and enabling transmigration into lymph nodes.
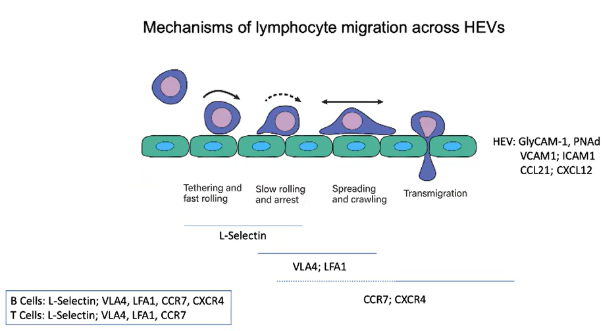
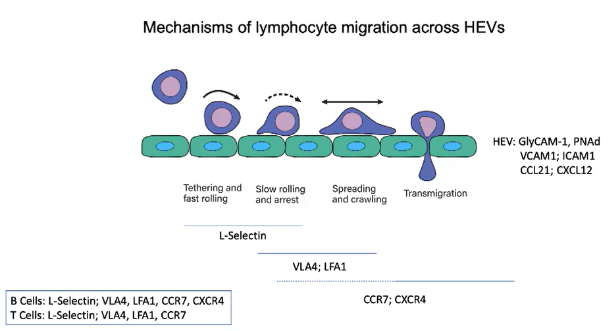
Describe the sequence of interactions that allow lymphocytes to enter lymph nodes through HEVs.
Rolling: L-selectin binds to GlyCAM-1 and PNAd.
Activation: CCR7 and CXCR4 receptors engage chemokines (CCL21 and CXCL12).
Arrest: Integrins VLA4 and LFA1 bind to VCAM1 and ICAM1.
Transmigration: Chemokine gradients guide lymphocytes into lymph nodes.
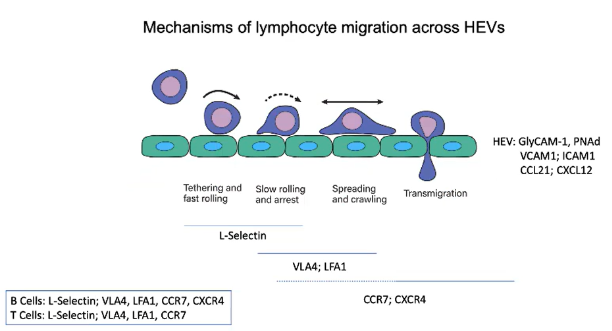
Which receptors do B and T cells share, and which are unique for lymph node migration?
Both B and T cells use:
L-selectin for initial rolling.
VLA4 and LFA1 integrins for adhesion.
CCR7 for CCL21 chemokine signaling.
B cells additionally use:
CXCR4 for CXCL12 chemokine signaling in lymph node migration.
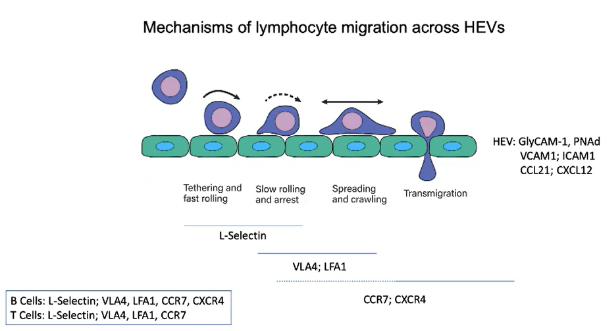
What role do addressins play in lymphocyte migration across HEVs?
Addressins (e.g., GlyCAM-1 and PNAd) are sticky proteoglycans expressed on HEVs.
Recognized by the L-selectin receptor on B and T cells.
As lymphocytes pass through HEVs, L-selectin binds to GlyCAM-1 and PNAd, creating a sticky interaction.
This interaction initiates tethering and fast rolling along the endothelial wall.
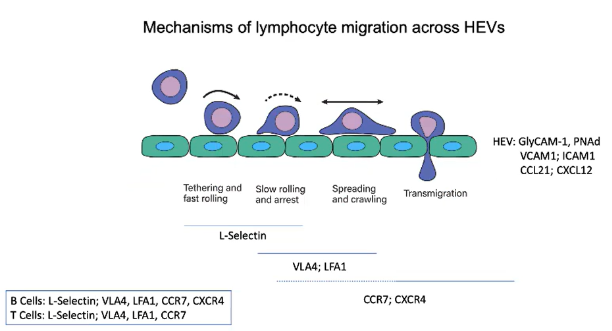
How do chemokines CCL21 and CXCL12 influence lymphocyte migration across HEVs?
HEV endothelial cells express chemokines CCL21 and CXCL12.
As lymphocytes roll through HEVs, they encounter these chemokines:
CCL21 engages with CCR7 receptor.
CXCL12 engages with CXCR4 receptor.
This receptor engagement activates integrins on the lymphocyte.
Integrins provide strong adhesion to secure the lymphocyte to the endothelial wall.
Prepares the lymphocyte for migration into the lymph node.
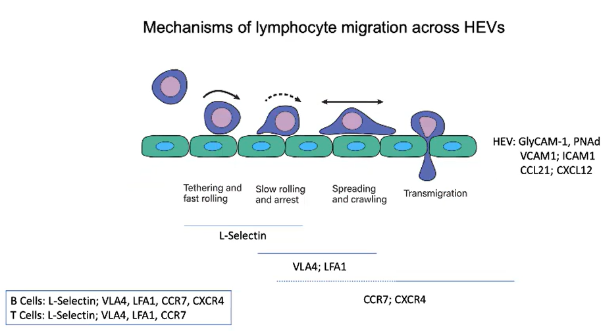
How do integrins and ligands regulate lymphocyte migration across HEVs?
Integrins VLA4 and LFA1 on B and T cells facilitate the shift from fast to slow rolling and eventual stopping on HEV endothelium.
GPCR signaling activates these integrins, shifting them from a dense to a tight conformation.
Activated integrins bind to their ligands:
VLA4 binds VCAM1.
LFA1 binds ICAM1.
This firm adhesion halts lymphocyte rolling, enabling response to chemokine signals.
Lymphocytes then migrate from the blood into the lymph node.
Chemokines within the lymph node guide B and T cells to their specific zones.
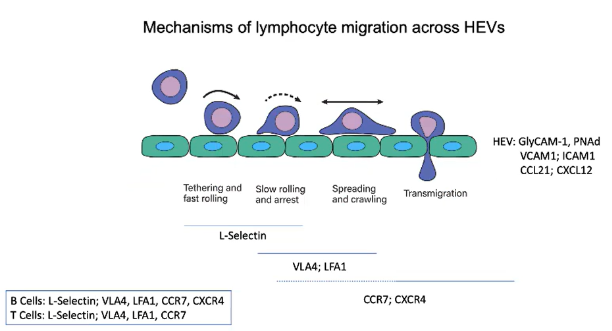
How do lymphocytes migrate through High Endothelial Venules (HEVs) to enter lymph nodes?
HEVs are unique blood vessels that express addressins (GlyCAM-1 and PNAd), making the endothelium "sticky".
B and T cells use L-selectin to bind to these addressins, initiating tethering and fast rolling on the endothelial wall.
HEVs also express chemokines CCL21 and CXCL12:
CCL21 binds to CCR7 on lymphocytes.
CXCL12 binds to CXCR4 on lymphocytes.
Chemokine binding activates integrins (VLA4 and LFA1) through GPCR signaling, shifting integrins to an active conformation.
Active integrins tightly bind to VCAM1 (for VLA4) and ICAM1 (for LFA1), stopping lymphocyte rolling.
This strong adhesion enables lymphocyte migration from the blood into the lymph node.
Within the lymph node, specific chemokines direct B and T cells to their respective zones.
How do T cells remain in the T cell zone of the lymph node?
After entering the lymph node, T cells remain in the T cell zone due to CCR7 expression.
CCR7 detects chemokines CCL21 and CCL19 in the T cell zone.
This zone has a fibroblast network specialized in expressing these chemokines.
CCL19 is soluble, while CCL21 is more membrane-bound.
The combination helps retain T cells within the T cell zone.
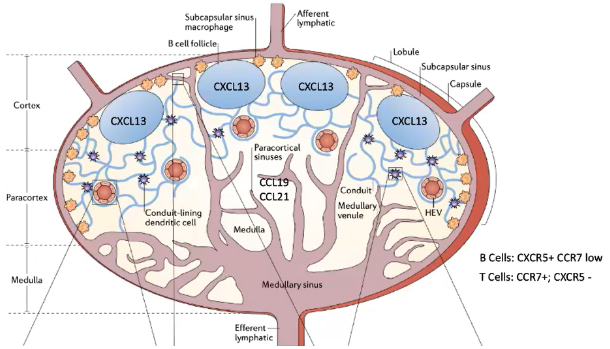
How do B cells localize to the B cell follicle in the lymph node?
B cells express low CCR7 but high CXCR5, which binds the chemokine CXCL13.
CXCL13 is produced by follicular dendritic cells (FDCs) in the B cell follicle.
Unlike T cell zones, the B cell follicle contains FDCs (fibroblasts, not hematopoietic dendritic cells).
FDCs display CXCL13 and preserve antigens on their surface.
This preservation allows B cells to recognize antigens without antigen destruction.
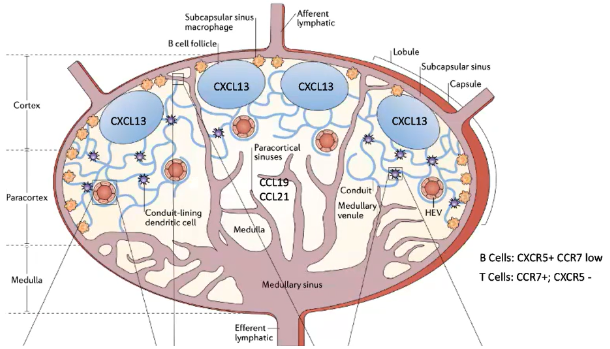
How do dendritic cells and lymphocyte receptors influence cell movement and antigen processing in the lymph node?
In the T cell zone, blood-derived dendritic cells process antigens and express CCR7.
These dendritic cells are exclusive to T cell zones and are not found in B cell follicles.
Each dendritic cell can interact with about 5,000 T cells.
B and T cells migrate between lymph nodes:
T cells express CCR7, with a half-life of ~12 hours in a lymph node.
B cells express CXCR5, with a longer half-life of ~24 hours in a lymph node.
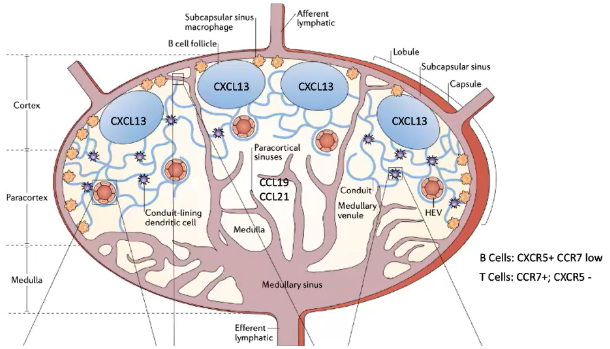
How do T cells and B cells remain in their respective zones within the lymph node, and how are antigens presented to them?
T Cells:
Remain in the T cell zone due to CCR7, which binds to CCL21 (membrane-bound) and CCL19 (soluble) from specialized fibroblasts.
Blood-derived dendritic cells in the T cell zone also express CCR7 and present processed antigens.
Each dendritic cell can interact with up to 5,000 T cells.
CCR7 allows T cells to stay in a lymph node for about 12 hours before migrating to another.
B Cells:
Have low CCR7 but high CXCR5, which binds CXCL13 from follicular dendritic cells (FDCs) in the B cell follicle.
Unlike T-cell-interacting dendritic cells, FDCs are fibroblast-derived and not hematopoietic.
FDCs preserve antigens on their surface, enabling B cell recognition without antigen destruction.
CXCR5 allows B cells to stay in a lymph node for around 24 hours before moving to another.
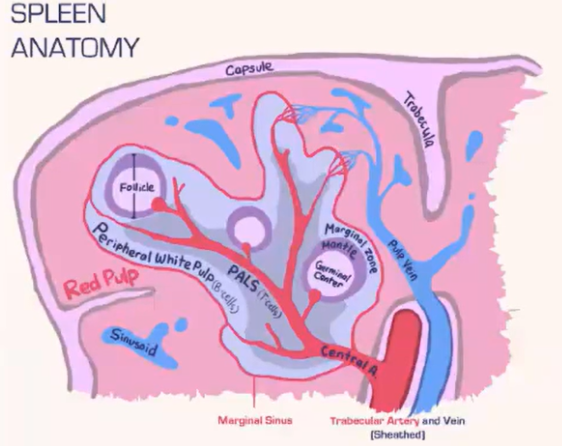
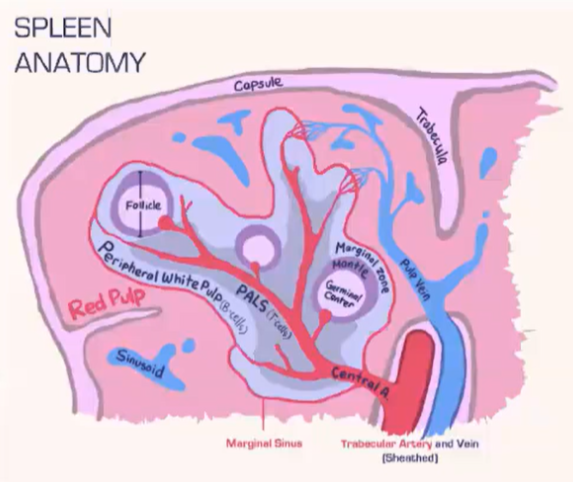
How does the spleen structure and function differ from lymph nodes, and how are T cells and B cells organized within it?
Spleen Structure:
Lacks a lymphatic system, relying solely on blood flow.
Consists of:
Red pulp: Contains red blood cells.
White pulp: Houses lymphocytes.
Blood is deposited at the marginal sinus (between red and white pulp), serving as a filter.
Lymphocyte Organization:
T Cells:
Move toward the T cell zone near the central arteriole.
Guided by CCR7 with support from fibroblastic reticular cells (FRCs).
B Cells:
Migrate to the B cell follicle in response to CXCR5.
Follicles contain follicular dendritic cells (FDCs), which preserve antigens for B cell recognition, similar to lymph nodes.
Lymphocyte Entry:
Lymphocytes passively enter the spleen, without selectins or rolling.
Integrins assist in adhesion at the marginal sinus area.
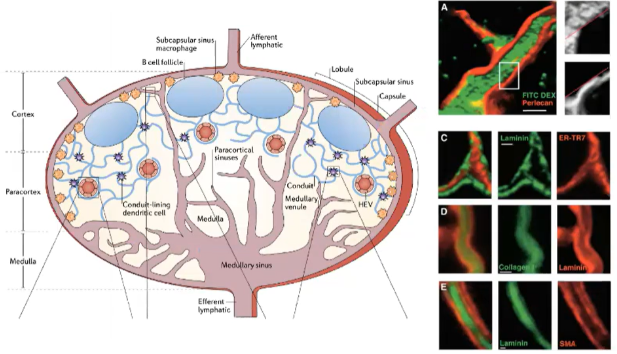
How are antigens channeled to different locations in the lymph node?
Small Antigens:
<60-70 kilodaltons (e.g., soluble proteins) are transported through a conduit system in the lymph node.
Conduits extend from the capsule into the T cell zone, allowing direct access for T cells.
Dendritic cells in the T cell zone pick up, process, and present these antigens on MHC molecules to T cells.
Large Antigens:
Captured by dendritic cells at the infection site.
Dendritic cells, with processed antigens, enter the lymph node via the afferent lymphatic system.
Present antigens to T cells within the T cell zone.
This system allows effective delivery of both small and large antigens to initiate an immune response in the lymph node.
How do conduits and dendritic cells contribute to antigen sampling in the lymph node T cell zone?
Conduits in the lymph node are narrow channels that exclude larger molecules.
Allow only small antigens (<70 kDa) to pass through.
Dendritic cells in the T cell zone continuously sample antigens from these conduits.
Enables constant surveillance of potential pathogens by dendritic cells.
What role do subcapsular sinus macrophages (SCS MΦ) play in antigen presentation to B cells within the lymph node?
Subcapsular sinus macrophages (SCS MΦ):
Located beneath the lymph node capsule near afferent lymphatic vessels.
Express complement receptors (e.g., CR1), allowing them to capture and retain opsonized antigens without internalizing or destroying them.
Displayed antigens provide B cells an opportunity for antigen encounter near the capsule.
Antigen Acquisition by B Cells:
B cells use CD21 (CR2) to "steal" opsonized antigens from SCS MΦ.
Interaction is independent of the B cell receptor (BCR) and relies solely on complement receptor engagement.
Antigen Transfer to Follicular Dendritic Cells (FDCs):
After acquiring the antigen, B cells carry it into the lymph node follicle.
B cells transfer the antigen to FDCs, which express CR1 (CD35) with high avidity, enabling strong antigen retention.
FDCs display antigens long-term, providing continuous exposure for B cells and supporting ongoing immune responses.
This pathway allows B cells to access and retain antigens without direct processing by traditional antigen-presenting cells.
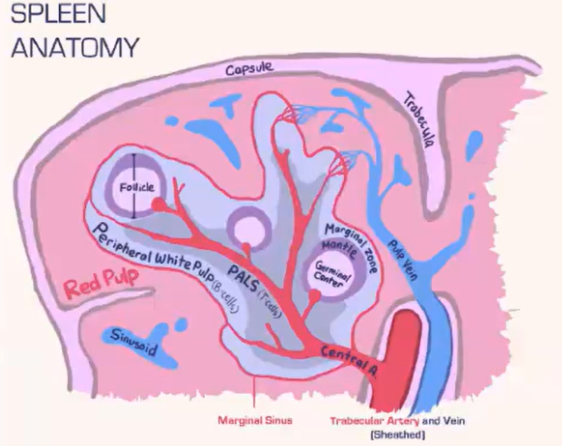
How does the spleen filter blood-borne pathogens, and what role do macrophages play?
The spleen has two main areas:
Red pulp: Contains red blood cells.
White pulp: Houses lymphocytes.
Marginal sinus separates red and white pulp, populated with phagocytic macrophages.
These macrophages filter blood-borne pathogens, acting as a primary defense.
Unlike lymph nodes, the spleen lacks non-phagocytic subcapsular sinus macrophages.
This structure enables the spleen to function as a primary blood pathogen filter.
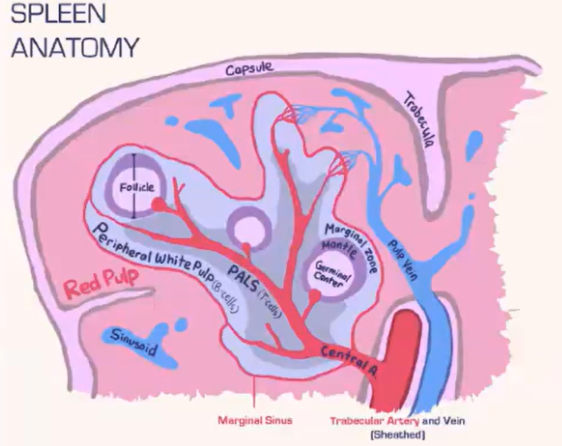
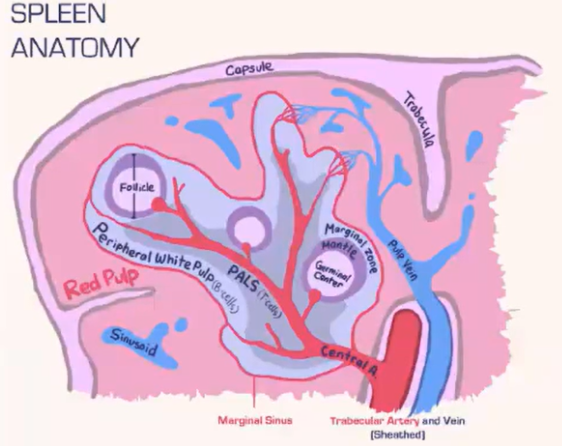
How do marginal zone B cells in the spleen aid in antigen presentation?
Marginal zone B cells are located beneath the marginal sinus in the B cell follicle.
They migrate between the red pulp and follicle approximately every 20 minutes.
Express high levels of CD21, efficiently capturing opsonized antigens missed by macrophages.
Movement is guided by S1P and CXCL13 gradients:
In the follicle, they sense S1P via S1PR1 and migrate to the S1P-rich red pulp.
In the red pulp, S1PR1 receptors internalize due to S1P saturation, guiding them back to the follicle via CXCR5 sensing CXCL13.
This cycle enables antigen deposition onto follicular dendritic cells (FDCs), supporting B cell immune responses against blood-borne pathogens.
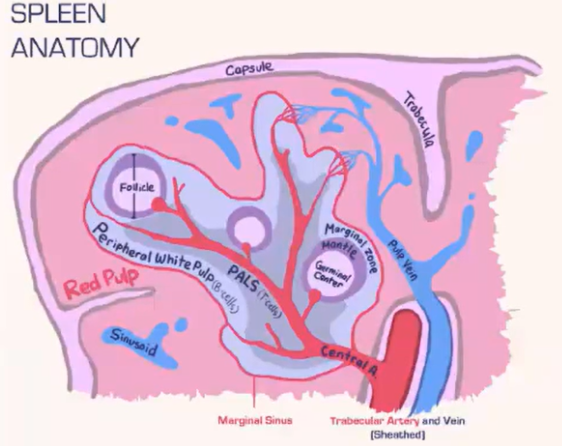
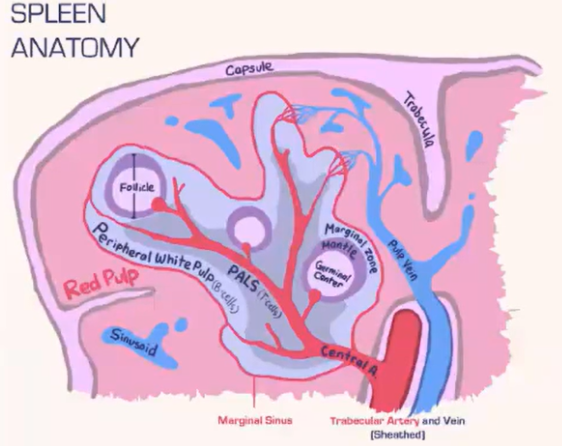
How do marginal zone B cells in the spleen contribute to antigen presentation?
Marginal zone B cells in the spleen's B cell follicle specialize in capturing opsonized antigens missed by macrophages.
Express high levels of CD21, efficiently picking up complement-bound antigens.
Migrate between the red pulp (S1P-rich) and the follicle (CXCL13-rich) approximately every 20 minutes, following S1P and CXCL13 gradients.
In the red pulp, high S1P levels cause S1PR1 internalization, directing cells back to the follicle using CXCR5 and CXCL13 signaling.
In the follicle, S1PR1 is re-expressed due to low S1P, guiding cells back to the red pulp.
During each cycle, they deposit captured antigens onto follicular dendritic cells (FDCs) in the follicle.
This process maintains a reservoir of antigens for presentation to B cells, supporting ongoing immune responses.