Alkenes
1/17
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
18 Terms
What are unsaturated hydrocarbons?
Hydrocarbons with a C=C bond
Why are alkanes vulnerable to attack by electrophiles?
The C=C bond consists of one pi bond and one sigma bond
Pi bonds are exposed and have high electron density
They attract electrophiles, which are electron deficient
What are electrophiles?
An electron pair acceptor
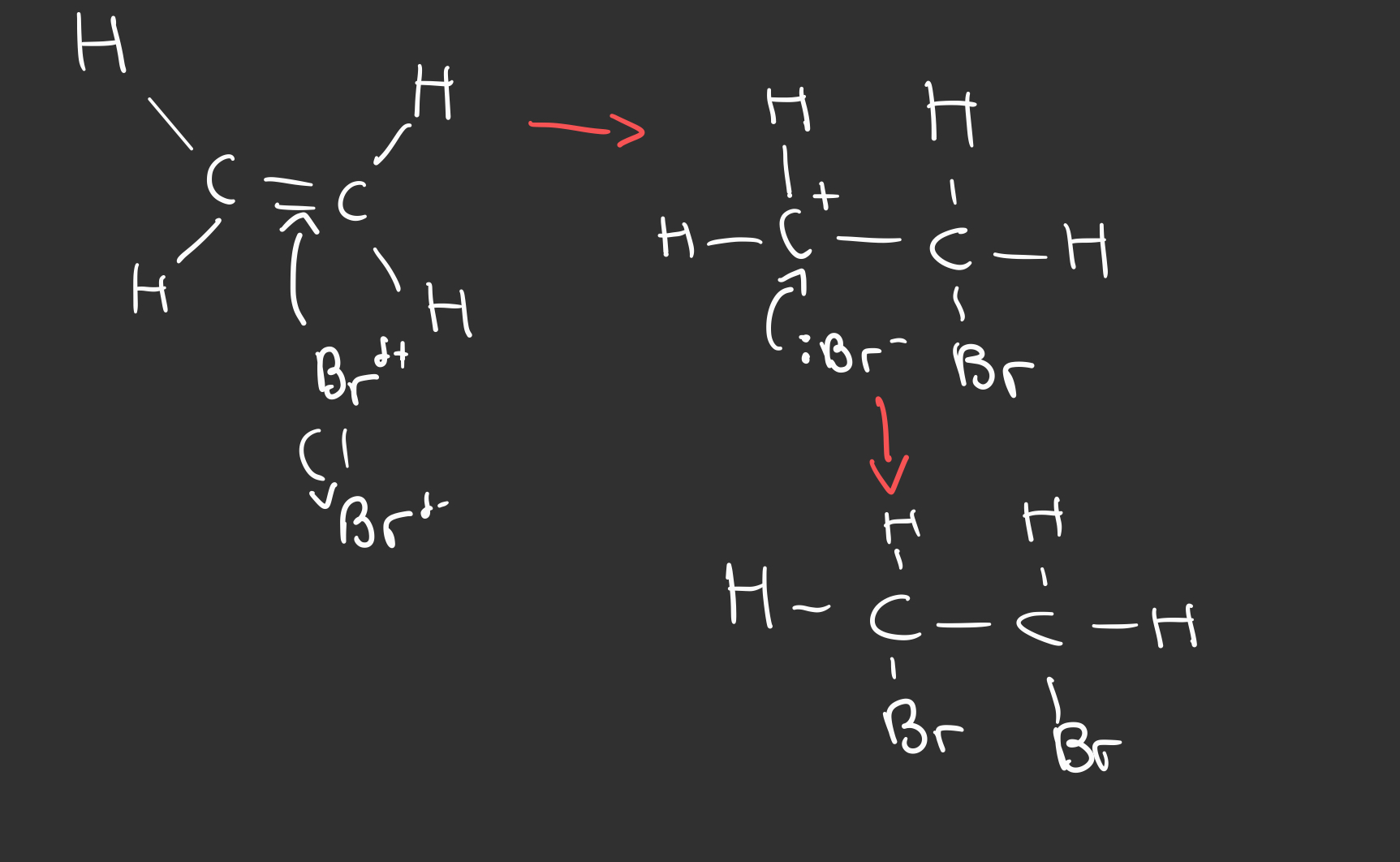
Outline the electrophilic addition reaction of bromine and alkenes
Outline the electrophilic addition reaction between HBr and alkenes
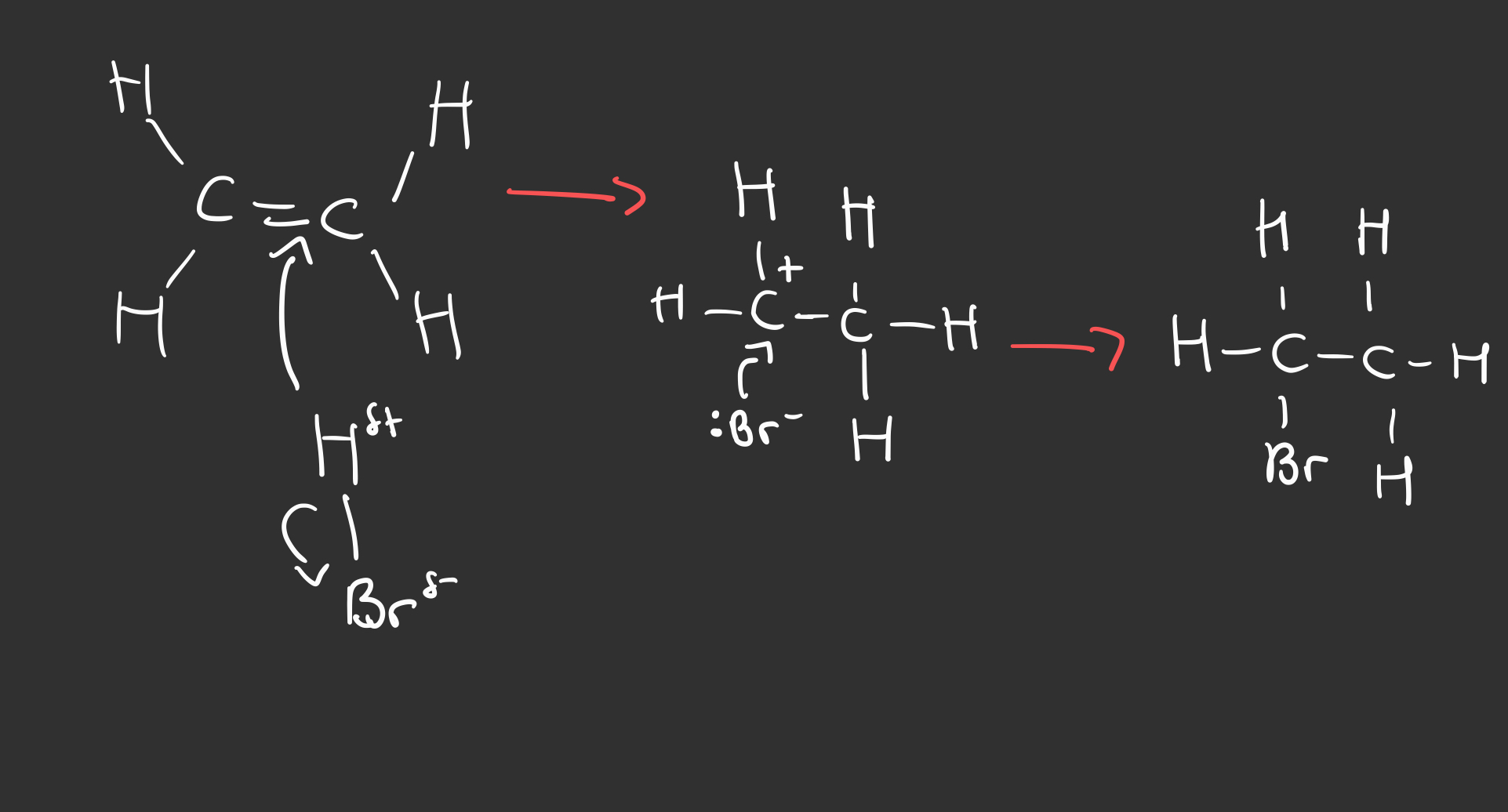
Which atom from the electrophile goes to the C+ ?
The atom with the negative partial charge
i.e. the more electronegative atom
When can 2 products from electrophilic addition form?
When the alkene is unstable
How do you know which product is major and minor?
The major product is formed via the more stable carbocation
How do you know if a carbocation is more stable or less stable?
Tertiary carbocations are most stable
Primary carbocations are least stable
Why are tertiary carbocations most stable?
They have the most amount of methyl groups that release electrons, reducing the charge on the carbocation, which stabilises it.
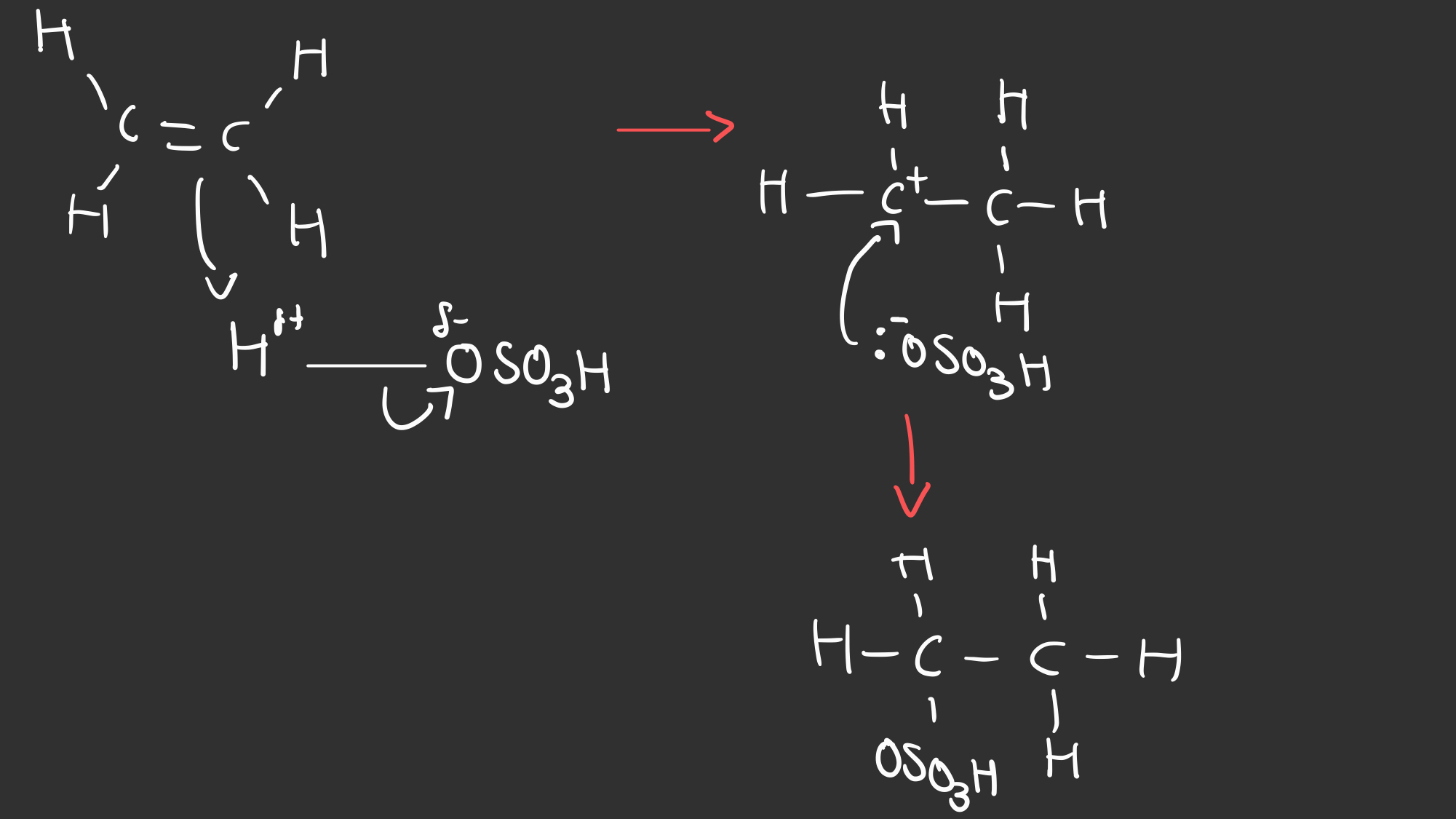
Outline the mechanism for the electrophilic addition reaction between H2SO4 and Alkenes
How can you test to see if an alkene was produced?
Add bromine water
Alkenes decolourise bromine water from orange to colourless
Outline how to draw the addition polymer from an alkene monomer
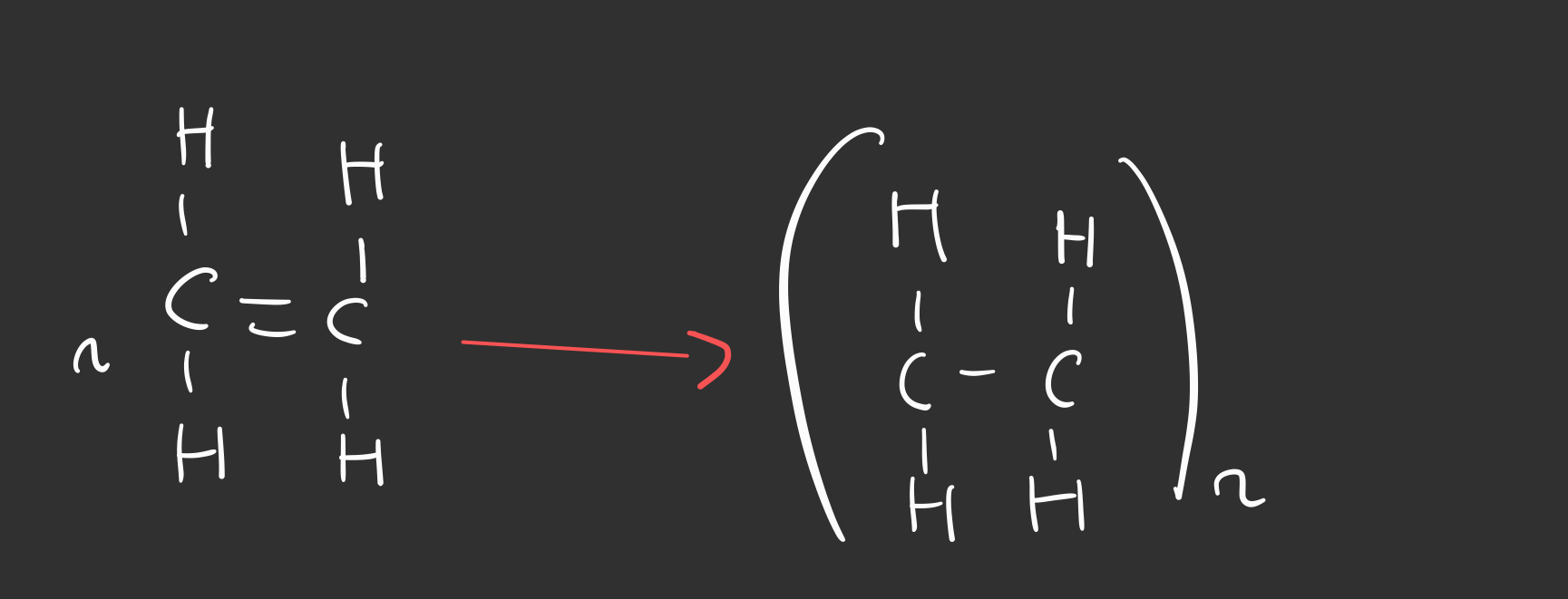
When do you add ‘n’ to the brackets?
If the question asks to draw ‘repeating units’
DON’T ADD ‘n’ if the question asks to draw one repeating unit
What are the uses of PVC?
Window frames coverings and gutterings
Why is PVC rigid in its pure form?
It has strong dipole-dipole forces between polymer chains from the C-Cl polar bond
Why is plasticiser added to PVC?
To weaken the dipole-dipole forces, so the chains can move more easily
Makes the polymer more flexible
What is a use of plasticised PVC?
Insulation for wiring