11 - sulphuric acid
0.0(0)
0.0(0)
Card Sorting
1/6
There's no tags or description
Looks like no tags are added yet.
Study Analytics
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
7 Terms
1
New cards
Lab preparation
S + 6HNO3 → H2SO4 + 6NO2 + 2H2O
2
New cards
contact process
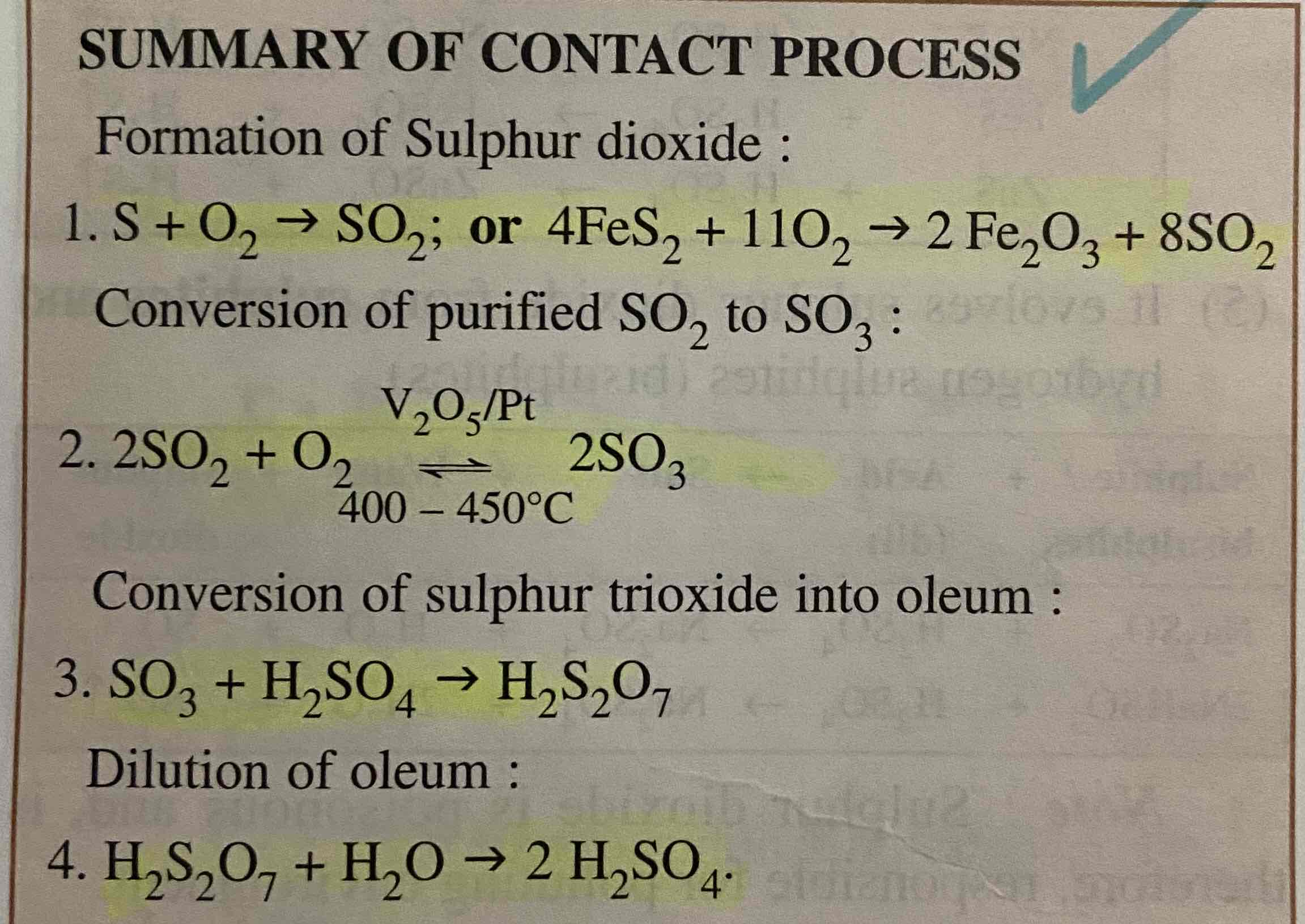
3
New cards
Name the catalyst used
Vanadium pentaoxide
4
New cards
Reaction: acidic property
Mg + H2SO4 → MgSO4 + H2
5
New cards
Reaction: Non volatile Nature
NaCl + H2SO4 → NaHSO4 + HCl
6
New cards
Reaction: As an oxidising agent
H2SO4 → H2O + SO2 + [O]
7
New cards
Reaction: Dehydrating agent
CuSo4.5H2O (Conc H2SO4) → CuSO4 + 5H2O
C12H22O11 (Conc H2SO4) → 12C + 11H2O
HCOOH (Conc H2SO4) → CO + H2O