Covalent bonds
1/50
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
51 Terms
covalent bonds
electrostatic attraction between a shared pair of electrons and the positively charged nuclei
electrons are shared rather than transferred
2 atomic orbitals overlap and molecular orbital is formed
electrons are more stable when attracted to 2 nuclei rather than one
electron pairs should be regarded as charge clouds, as they are in a state of constant motion
octet rule
tendency of atoms to gain a valence shell with a total of 8 electrons
being able to accommodate more than 8 electrons in the outer shell - expanding the octet
accommodating less than 8 electrons - electron deficient
steps for drawing lewis structures
count total number of valence
draw skeletal structure
use dots/crosses to show an electron pair
add more electron pairs to complete the octets around the atoms
if not enough electrons to complete octet, add double/triple bonds
incomplete octet examples
BeCl2, BF3
Be and B form stable compounds with 4 and 6 valence electrons respectively
bond energy
energy required to break one mole of a particular covalent bond in the gaseous states, the larger the bond energy, the stronger the covalent bomd
bond length
internuclear distance of 2 covalently bonded atoms
the greater the forces of attraction are, the more the 2 atoms are pulled to eachother
this decreases the bond length of a molecule and increases the strength of a covalent bond
triple bonds are the shortest and strongest due to large electron density between the nuclei
coordinate bonds
some molecules have a lone pair of electrons that can be donated to form a bond with an electron deficient atom
both electrons share the same atom
this is called coordinate bonding, use an arrow to indicate it
e.g ammonium ion donating to H+ ion
VSEPR theory
when an atom forms a covalent bond with another atom, the electrons in the different bonds and the non-bonding electrons in the outer shell all behave as negatively charged clouds, repelling eachother
regions of negative cloud charge are known as domains,
in order to minimise this repulsion, all outer shell electrons are spread out as far as possible in the space
molecular shapes and their angles can be predicted through this theory
3 rules of VSEPR theory
all electron pair and all lone pairs arrange themselves as far apart in the space as possible
lone pairs repel more strongly than bonding pairs (because they’re pulled more closely to the central atom)
multiple bonds behave like single bonds
2 electron domains
shape: linear
bond angle: 180
3 electron domains
shape: trigonal planar
bond angle: 120
if one of these domains is a lone pair, bond angle is slightly less than 120 (118) due to the stronger repulsion from lone pairs, forcing the bonding pairs closer together
this shape is now called: bent linear
4 electron domains
shape: tetrahedral
bond angle: 109.5
if one of the electron domains is a lone pair, the bond angle is slightly less than 109.5 (107)
this shape is now called: trigonal pyramidal
if two of these electron domains is a lone pair, the bond angle is slightly less than 109 (104.5)
this shape is now called: bent linear
electronegativity
the ability of an atom to draw electrons towards itself in a covalent bond
in diatomic molecules, electron density is shared between the 2 atoms
both atoms have the electronegativity value, and have an equal attraction for the bonding pair of electrons, this forms a covalent bond
polar bond
when 2 atoms in a covalent bond have 2 different electronegativities, the covalent bond is polar and the electron is drawn to the more electronegative side
electron distribution is asymmetric
extent of polarity in a covalent bond varies, depending on how big a difference in electronegativity values between the 2 atoms
the higher the difference, the higher the polarity
dipole moment
measure of how polar a bond is
direction of the dipole movement is shown by an arrow, pointing towards the partially negative charged end of the pole

molecular polarity
to determine whether a molecule with more than 2 atoms is polar consider:
polarity of each bond
geometry of the molecule
some molecules have polar bonds, but are overall non-polar because the molecule is arranged in such a way that the dipole moments cancel eachother out
covalent lattices
in some cases it is impossible to satisfy the bonding capacity of a substance in the form of a molecule, the bonds between atoms continue indefinitely and a large lattice is formed
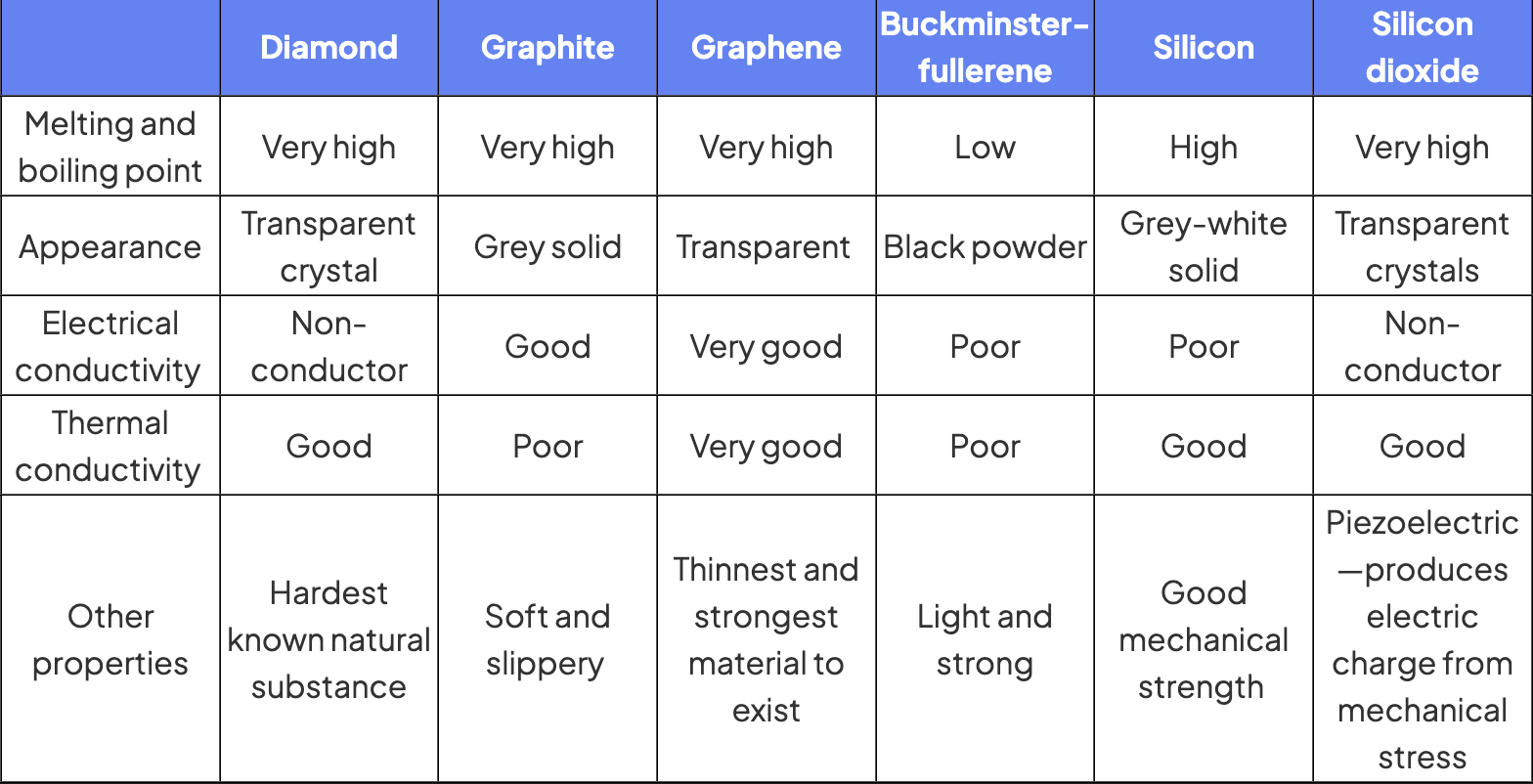
these are called giant covalent structures, most important examples are C and SiO2
allotrope
different molecular arrangements of the same element in the same physical state
diamond
each carbon to 4 others tetrahedral arrangement with a bond angle of 109.5
results in giant lattice with strong bonds in all directions
graphite
each carbon atom bonded to 3 others in a layered structure
layers made of hexagons with bond angle of 120
spare electron is delocalised and occupies space between layers
all atoms in the same layer held together by strong covalent bonds, different layers held together by weak IM forces
buckminsterfullerene
contains 60 carbon atoms, each is bonded to 3 others by covalent bonds
4th electron is delocalised so electrons can migrate throughout the structure, making it a semi conductor
graphene
made of single layer of carbons bonded together in a repeating pattern of hexagons
silicon
each silicon atom bonded to 4 others, tetrahedral arrangement
silicon (IV) oxide
each silicon bonded to 4 oxygen atoms, each oxygen is shared by 2 silicon atoms tetrahedral arrangement
tetrahedral units are repeated
charateristics of giant covalent structures
intermolecular forces
forces of attraction between molecules in molecular covalent compounds
three main types:
london forces
dipole dipole attraction
hydrogen bonding
london dispersion forces
electrons are in a state of constant motion, therefore it is likely they are not always distributed symmetrically
this is known as a temporary dipole
constantly appearing and disappearing as electrons keep moving
an adjacent atom will be repelled and attracted by the dipole and will move accordingly
this is a temporary induced dipole
london forces are present between all molecules but are generally very weak (weakest out of IM bonds)
happens between non-polar species
strength of the forces depends on:
number of electrons in molecule
surface area of molecule
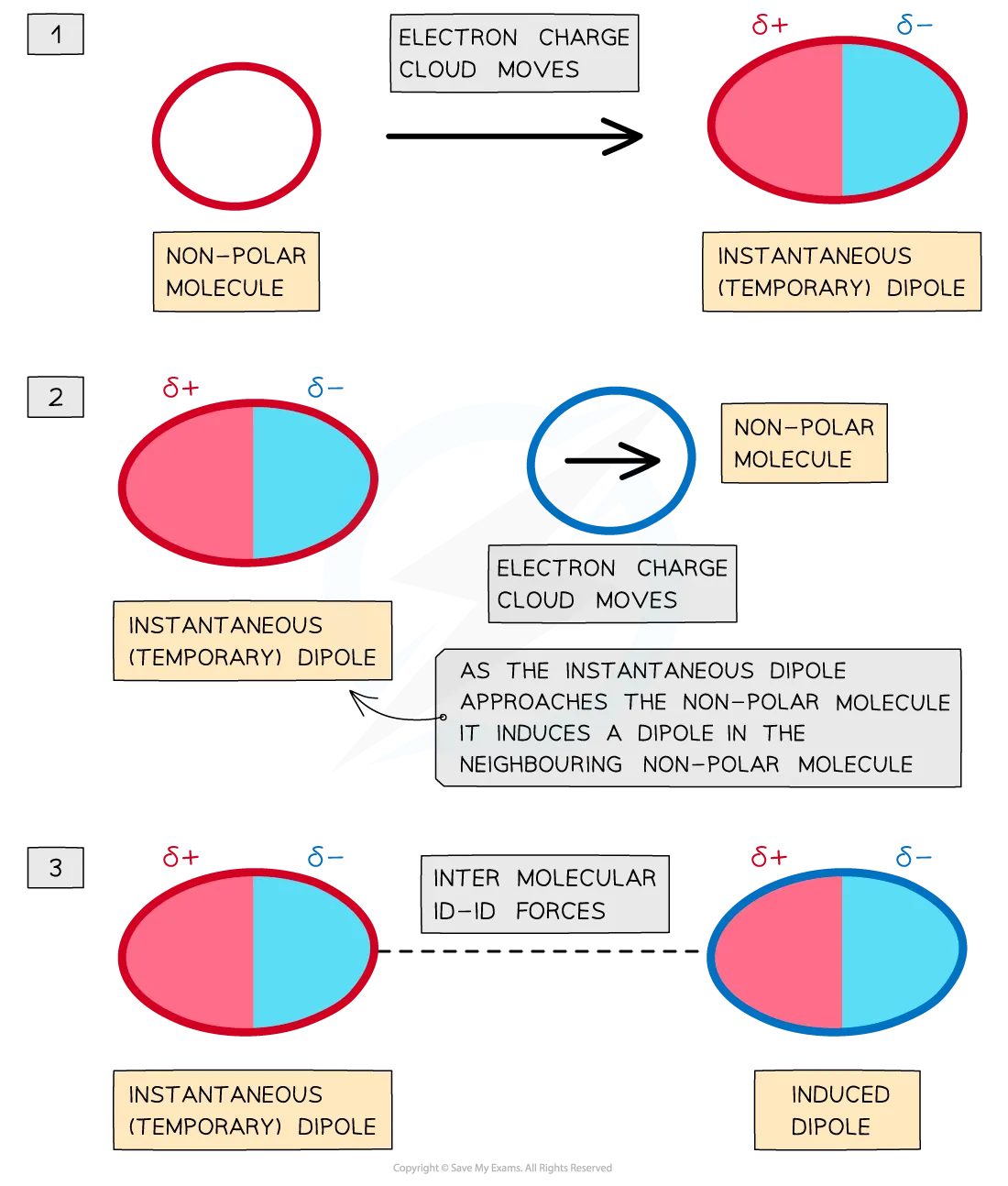
number of electrons (london forces)
the more electrons in a molecule, the greater possibility of a distortion, thus the greater frequency and magnitude of temporary dipoles
london forces are stronger and mpt/bpt is higher
surface area (london forces)
the larger the SA, the larger contact with adjacent molecules
the greater the ability to induce a dipole in an adjacent molecule, the greater the london forces and the higher the mpt/bpt
dipole-dipole attractions
temporary dipoles exist in all molecules, in some molecules there is also permanent dipole
in addition to london forces caused by temporary dipoles, molecules with permanent dipoles are also attracted to eachother
this type of bonding slightly increases bpt than would be expected with just london forces, as well as strength of IM bonds
second weakest out of IM bonds
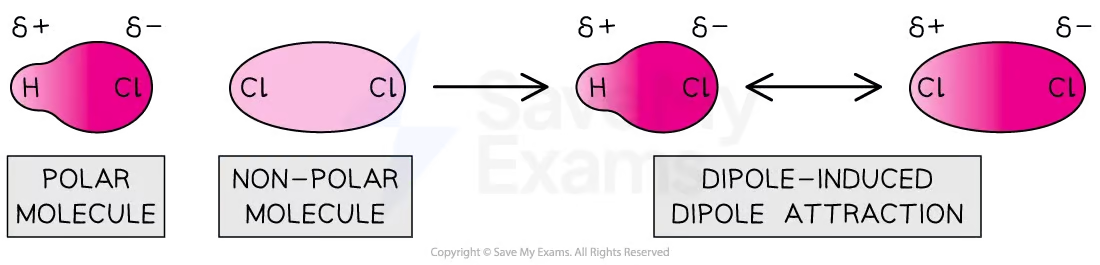
dipole induced dipole attraction
some mixtures contain both polar and non polar molecules
the permanent dipole of the polar molecule can cause a temporary separation of charge in a non-polar molecule
this force acts in addition to london forces between non polar molecules, and dipole dipole attraction in polar molecules
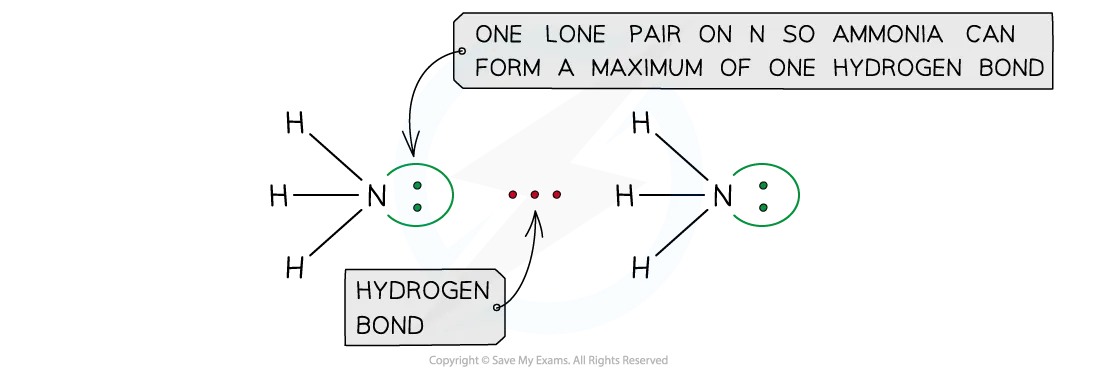
hydrogen bonding
strongest type of IM force
for hydrogen bonding to take place:
a species which has an O, N , or F (very electronegative) atom, with an available lone pair
a hydrogen attached to the O, N or F
when this happens, the bond becomes very polarised
the H becomes so delta positive that it can form a bond with a lone pair of O, N, F in another molecule
hydrogen bonds represented by dashes
van der Waals forces
used to refer to
london dispersion forces
dipole dipole attraction
dipole induced dipole attraction
Hydrogen bonding
melting and boiling point explained in covalent
when covalent molecular substances change state, you are overcoming IM forces
the stronger the forces, the more energy needed to break
IM forces are much weaker than covalent bonds, this is why many covalent substances are liquid/gas at rtp
volatility is high when mpt/bpt is low
solubility explained in covalent
non-polar dissolves in non-polar solvents, london forces form between solvent and solute
polar dissolves in polar solvents as a result of dipole-dipole attraction, or hydrogen bonding between solvent and solute
as covalent molecules become larger, solubility can decrease as polar part of molecule is a smaller part of overall structure
this is why giant covalent structures are generally insoluble
conductivity explained in covalent
as covalent substances contain no mobile charged particles, they’re unable to conduct in solid or liquid state
some giant covalent structures can conduct due to delocalised electrons, but are exceptions
Rf values in chromatography
the extent of separation of the component molecules in the investigated sample depends on their solubility in the mobile phase and the extent of adhesion to the stationary phase
Rf values are used to quantify the distance travelled relative to solvent front

resonance structures
delocalisation of electrons explains why structures of some species don’t fit with lewis formula
delocalised electrons are electrons in a molecule that aren’t associated with a single covalent bond or atom
the lewis structure for nitrate ion has a double bond and 2 single bonds
there are 3 ways to structure this molecule
these structures are resonance structures
although, bonds are all equal in length and electron density is spread evenly between the 3 oxygen atoms
the bond length is somewhere between a single and a double bond, and the structure is somewhere in between the 3, this is called a resonance hybrid
dotted lines are used to show the delocalised electrons
criteria for resonance structures
molecules must have a double bond that is capable of migrating from one part of the molecule to another
lone pairs of electrons that can re-arrange themselves and allow the double bonds to be in different positions
benzene as a resonance structure
each carbon atom in the ring forms 3 σ bonds using the sp2 orbitals
the remaining p orbitals overlap laterally w/ p orbitals of neighbouring carbons forming a π system
extensive side ways overlap allows delocalisation of electrons, being able to spread freely around the ring.
delocalisation of electrons renders the carbon-carbon bonds to have both double and single bond character (resonance).
evidence includes:
bond length being between the single and double value
undergoes substitution reactions instead of addition reactions
expansion of the octet
elements in period 3 and above can have more than 8 electrons in valence shell, because of the d-subshell being able to accommodate more electron pairs
5 electron domains
5 BP 0 LP: trigonal bimpyramidal,
4 BP 1 LP: see saw
3 BP 2 LP: t- shape
2 BP 3 LP: linear
6 electron domains
6 BP 0 LP: octahedral
5 BP 1 LP: square based pyramid
4 BP 2 LP: square planar
formal charge
sometimes it’s difficult to determine which lewis structure is most appropriate for a molecule
formal charge is the charge assigned to an atom in a molecule, assuming that all the electrons in the bonds are shared equally between atoms, regardless of differences in electronegativity
FC= (number of valence electrons) - ½(number of bonding electrons) - (number of non-bonding electrons)
the lewis formula that is preferred is:
the difference in FC of the atoms is closest to zero
negative charges located on most electronegative atoms
bond overlap
each atom that combines has an atomic orbital with an unpaired electron
when 2 atomic orbitals overlap, they form a molecular orbital containing 2 electrons
the greater the overlap, the stronger the bond
sigma bonds
formed from head on overlap of atomic orbitals
electron density is concentrated along the bond axis (imaginary line between the 2 nuclei)
single covalent bonds are always sigma bonds
can be s+s, s+p or p+p
pi bonds
sideways overlap of adjacent p orbitals
the 2 lobes that makeup the pi bond are above and below the plane of the sigma bond
the electron density is concentrated on opposite sides of the bond axis
only found within double and triple bonds
hybridisation
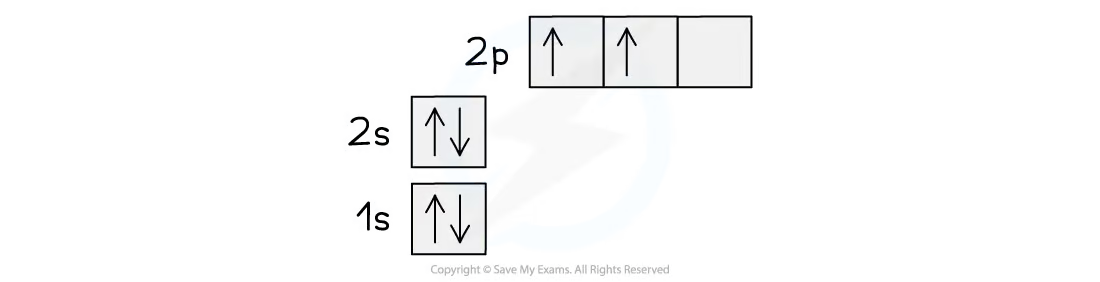
the electronic structure of the ground state of carbon would imply that it uses the unpaired 2p electrons to form covalent bonds
this is not true, as carbon usually forms 4 covalent bonds
a half full p-subshell is slightly lower in energy than a partially filled one
the difference in energy between the 2s and the 2p subshell is small, so an electron can be promoted fairly easily from the 2s to the 2p
the 2s and the 2p subshells blend together to form 4 new hybrid orbitals (because 1 s and 3 p)
this would give 4 unpaired electrons capable of forming 4 covalent bonds
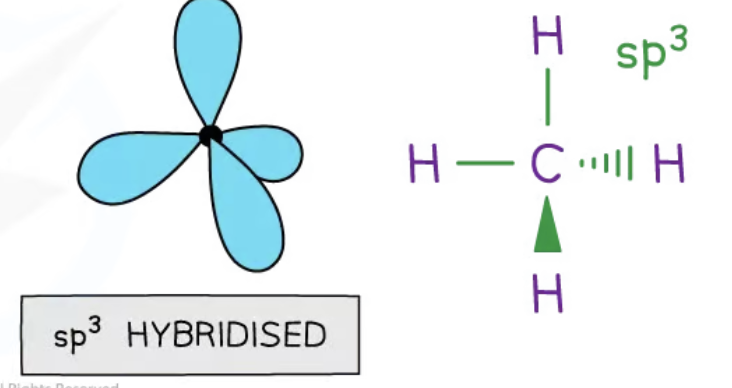
sp3 hybridisation
4 hybrid orbitals are produced when the 2s and and three 2p are blended together
these hybrids are 25% s character and 75% p character
the 4 sp3 orbitals space themselves out at 109.5 degrees in a tetrahedral shape
carbon atom forms single bonds
sp3 orbitals merge with s orbitals in hydrogen forming 4 equal sigma bonds
lone pairs can also be present in hybridisation e.g in ammonia
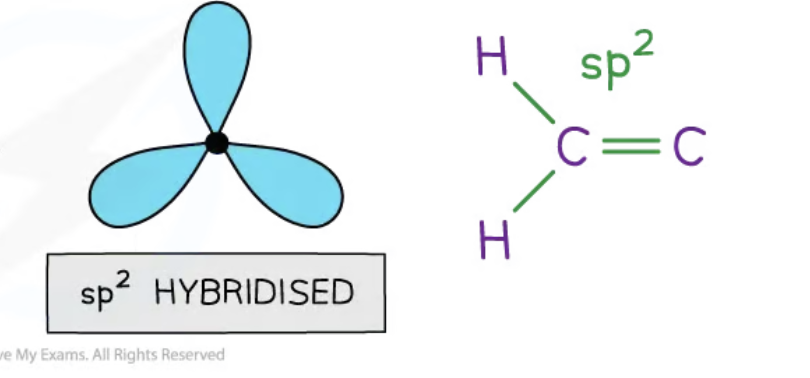
sp2 hybridisation
3 hybrid orbitals are produced when the 2s and two 2p are blended together
these hybrids are 1/3 s character and 2/3 p character
space themselves out at 120 degrees, in a trigonal planar shape
carbon atom forms double bond
sp2 orbital merges with s orbitals in hydrogen, and the sp2 of an adjacent carbon molecule, forming 3 equal sigma bonds
double bond is created by sideways overlap of the unhybridised p orbitals, causing formation of 1 pi bond
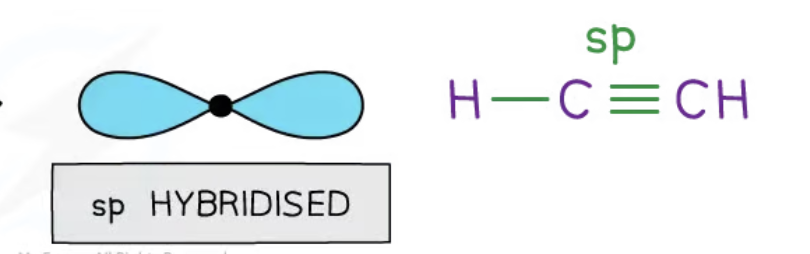
sp hybridisation
2 hybrid orbitals are produced when the 2s and one 2p are blended together
these hybrids are ½ s character and ½ p character
space themselves out at 180 degrees, in a linear shape
the carbon atom forms a triple bond
sp orbital merges with s orbital in hydrogen, and the sp of an adjacent carbon to form 2 equal sigma bonds
triple bond is created by head-on overlap of 2 pairs of unhybridised p orbitals (from the 2 carbons)